Mass transfer¶
- Combustion involves many chemical species.
- A rigorous treatment of mass transfer is often important.
Velocity¶
- If we could see a molecular view of a flow, we would not see a "velocity," we would see many molecules, each with their own velocity.
- If we consider the average velocity of a particular species, we can define average velocities
- Mass average
- Mole average
- Volume average
Mass and mole flux¶
- Total mass flux for a mass average velocity $$\dot{m}^{\prime\prime} = \rho v$$
- Species $A$ $$\dot{m}^{\prime\prime}_A = \rho y_A v_A$$
- Total molar flux for a molar average velocity $$\dot{n}^{\prime\prime} = c v$$
- Species $A$ $$\dot{n}^{\prime\prime}_A = c x_A v_A$$
Diffusion velocity¶
$$v_A = v_A + v-v$$ $$v_A = v + \underbrace{(v_A - v)}_{v_A^{D}}$$
- Mass flux $$\dot{m}^{\prime\prime}_A = \rho y_Av_A = \rho y_Av + \rho y_Av_A^{D}$$
- This last expression is (Bulk flux of A) + (Diffusion flux of A)
- $j_A$ is the diffusion flux.
- Now, add all species: $$\dot{m}^{\prime\prime} = \sum_i\dot{m}^{\prime\prime}_i = \sum_iy_i\dot{m}^{\prime\prime}+\sum_ij_i = \dot{m}^{\prime\prime}\sum_iy_i + \sum_ij_i = \dot{m}^{\prime\prime} + \sum_ij_i $$
- The diffusion flux is a difference from an average flux. So, the sum of all diffusion fluxes should be zero.
Multicomponent mass transfer¶
- Model the $j_i$ as gradients of species.
- Using a mole basis is more physical.
- $J_i$ is the molar flux of species $i$
- Fluxes depend on mole fraction gradients.
Two species 1, 2¶
$$J_1 = -cD\nabla x_1,$$ $$J_2 = -cD\nabla x_2.$$
Three species 1, 2, 3¶
$$J_1 = -cD_{1,1}\nabla x_1 - cD_{1,2}\nabla x_2,$$ $$J_2 = -cD_{2,1}\nabla x_1 - cD_{2,2}\nabla x_2.$$
- The flux of one species depends on its own gradient and on the gradient of other species.
- Other species can drag on each other.
- Species can diffuse up their gradients!
- Question why do we not include $\nabla x_3$ in $J_1$ above?
Matrix form¶
$$\mathbf{J} = -c[D]\mathbf{\nabla x},\phantom{xxxxxx}\text{size = }n_s-1$$- $[D]$ is a diffusion matrix defined as follows
$$B_{i,j} = -x_i\left(\frac{1}{\mathcal{D}_{i,j}} - \frac{1}{\mathcal{D}_{i,n_s}}\right),\phantom{xxx} i\ne j$$
$$B_{i,i} = \frac{x_i}{\mathcal{D}_{i,n}} + \sum_{k=1,i\ne k}^{n_s} \frac{x_k}{\mathcal{D}_{i,k}}$$
- $\mathcal{D}_{i,j}$ are binary diffusion coefficients.
- Available in tables. See Turns Appendix D.
- Cantera can provide these also.
- $\mathcal{D}_{i,j} \propto T^{3/2}/P$
Fick's law¶
- Beware, there are various forms of "Fick's" law.
- We will use the "best" one
- $D_{i,e}$ is an effective diffusivity
- To get mass flux, multiply by $M_i$
$$j_i = M_iJ_i = -M_icD_{i,e}\nabla x_i$$
- Use $c=\rho/M$, and $x_i = y_iM/M_i$
$$ j_i = -\frac{M_i\rho}{M}D_{i,e}\frac{M}{M_i}\nabla y_i - \frac{M_i\rho}{M}D_{i,e}\frac{y_i}{M_i}\nabla M$$
$$j_i = -\rho D_{i,e}\nabla y_i - \left(\rho D_{i,e}y_i\frac{\nabla M}{M}\right)$$- The second term, in parentheses is often ignored.
- Not a great assumption for combustion.
- Often used in combustion models for turbulent flow.
Note: $J_i$ is relative to a molar average velocity. So, our conversion to $j_i$ and the use of a mass average velocity is not fully consistent.
Note: In combustion, we often have lots of $N_2$ and using Fick's law instead of a full multicomponent treatment is not that bad.
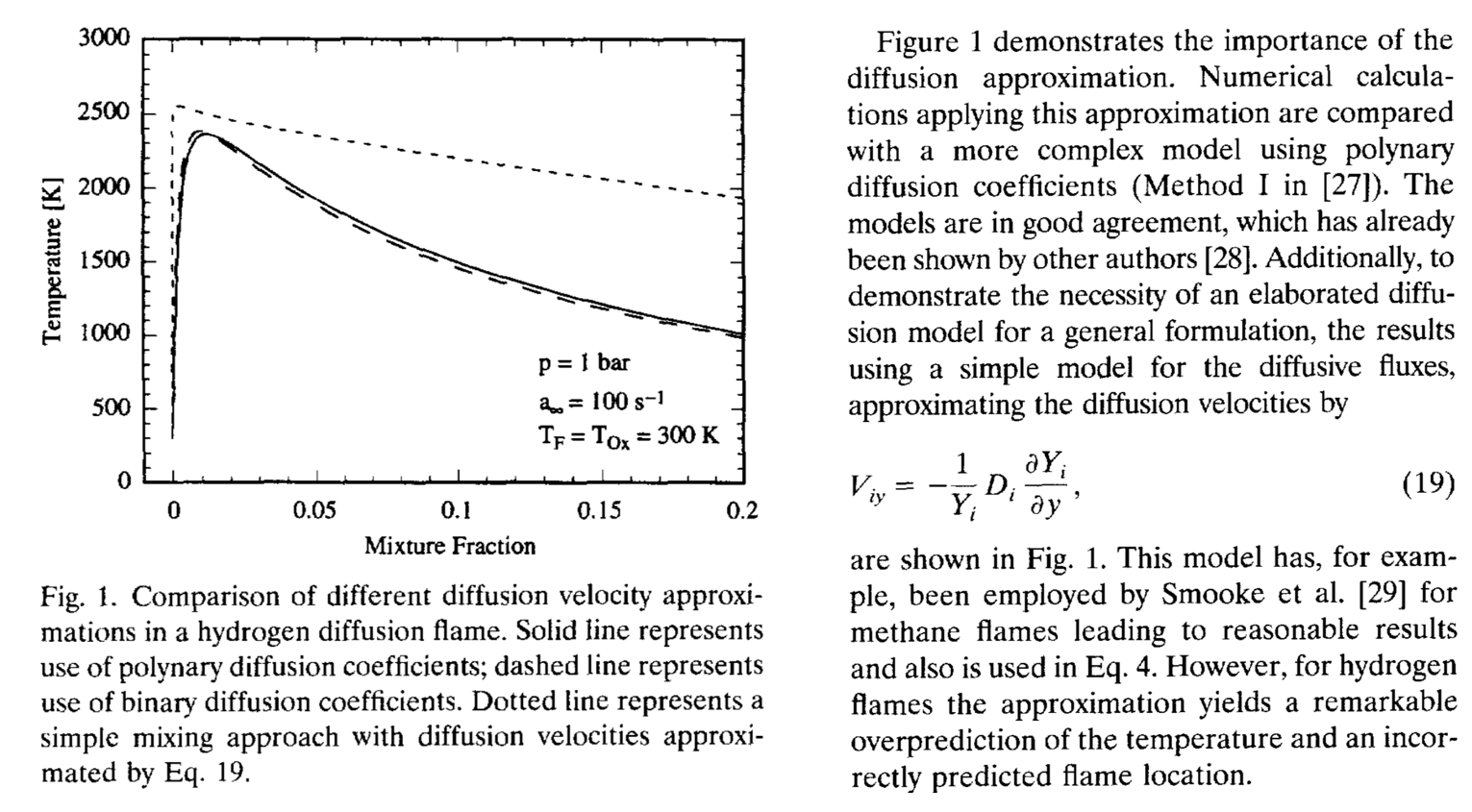
$$D_{i,e} = \left[\frac{(1-x_i)}{\sum_{j=1,j\ne i}^n \frac{x_j}{\mathcal{D}_{i,j}}}\right]$$The following illustrates the importance of using the "full" Fick's law form given above. This is from Pitsch and Peters (1998).
Heat, Mass, Momentum¶
- Mass transfer: $$\rho y_i \rightarrow j_i = -\rho D\nabla y_i$$
- Heat transfer: $$\rho h \rightarrow q = -\lambda \nabla T$$
- Momentum transfer: $$\rho u \rightarrow \tau = -\mu \nabla v$$
Also, $$ y_i \rightarrow -D\nabla y_i$$ $$ T \rightarrow -\alpha\nabla T$$ $$ v \rightarrow -\nu\nabla v$$
$\alpha = \lambda/(\rho c_p)$
$\nu = \mu/\rho$
$D$, $\alpha$, $\nu$ all have units of $m/s^2$
For constant properties, the unsteady diffusion equation for some scalar $\eta$, with diffusivity $\Gamma$ is $$\frac{\partial \eta}{\partial t} = \Gamma \frac{\partial^2\eta}{\partial x^2}$$
Dimensionless numbers¶
- Lewis number $$ Le = \frac{\alpha}{D}$$
- Schmidt number $$ Sc = \frac{\nu}{D}$$
- Pr number $$ Pr = \frac{\nu}{\alpha}$$
One-dimensional species transport equation¶
$$\frac{\partial \rho y_i}{\partial t} + \frac{\partial}{\partial x}(\rho y_i v_i) = S_i$$
$$v_i = v + v_i^D$$ $$\rho y_i v_i^D = j_i$$
$$\frac{\partial \rho y_i}{\partial t} + \frac{\partial}{\partial x}(\rho y_i v) + \frac{\partial j_i}{\partial x} = S_i$$