ChEn 433 Nuclear
Class 16-17
Nuclear
What are the major issues associated with nuclear power?
- No GHG emissions (CO\(_2\))
- Environmental concerns
- Safety concerns
- Proliferation
- Waste storage
- Fuel resources
- Regulation
- Public perception
Nuclear timeline

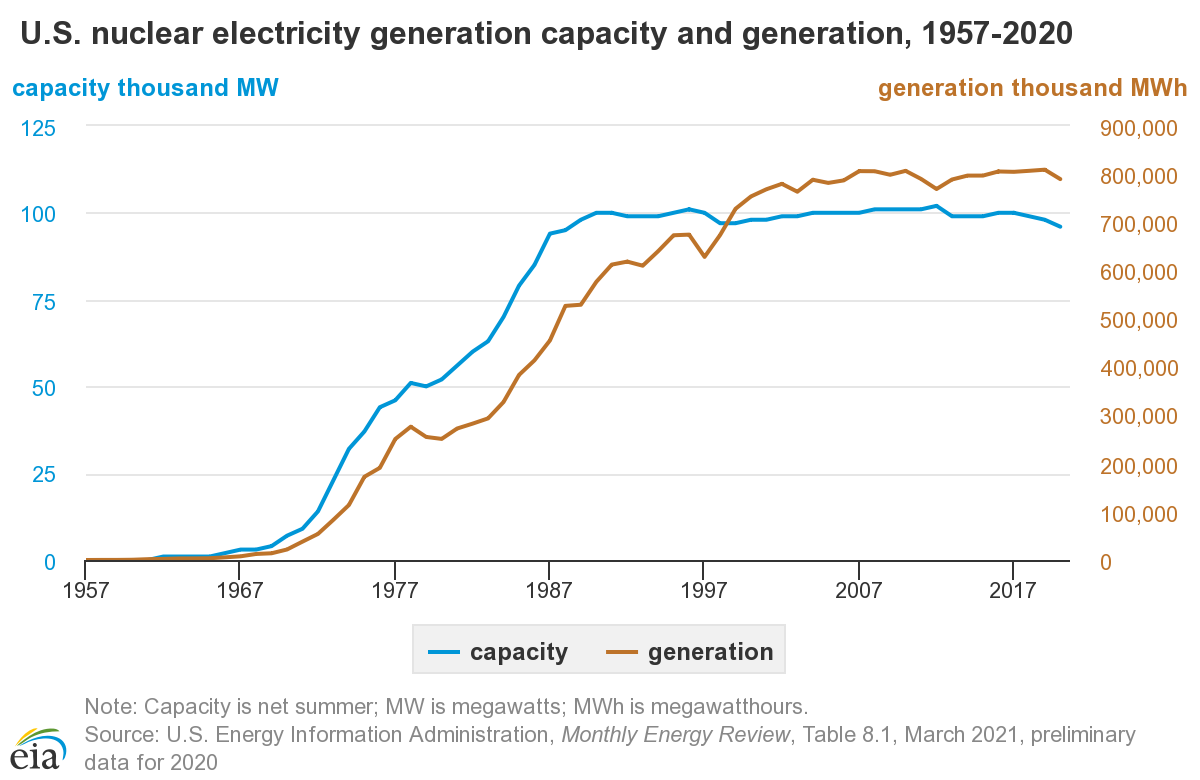
U.S. nuclear power by year
U.S. nuclear plant locations

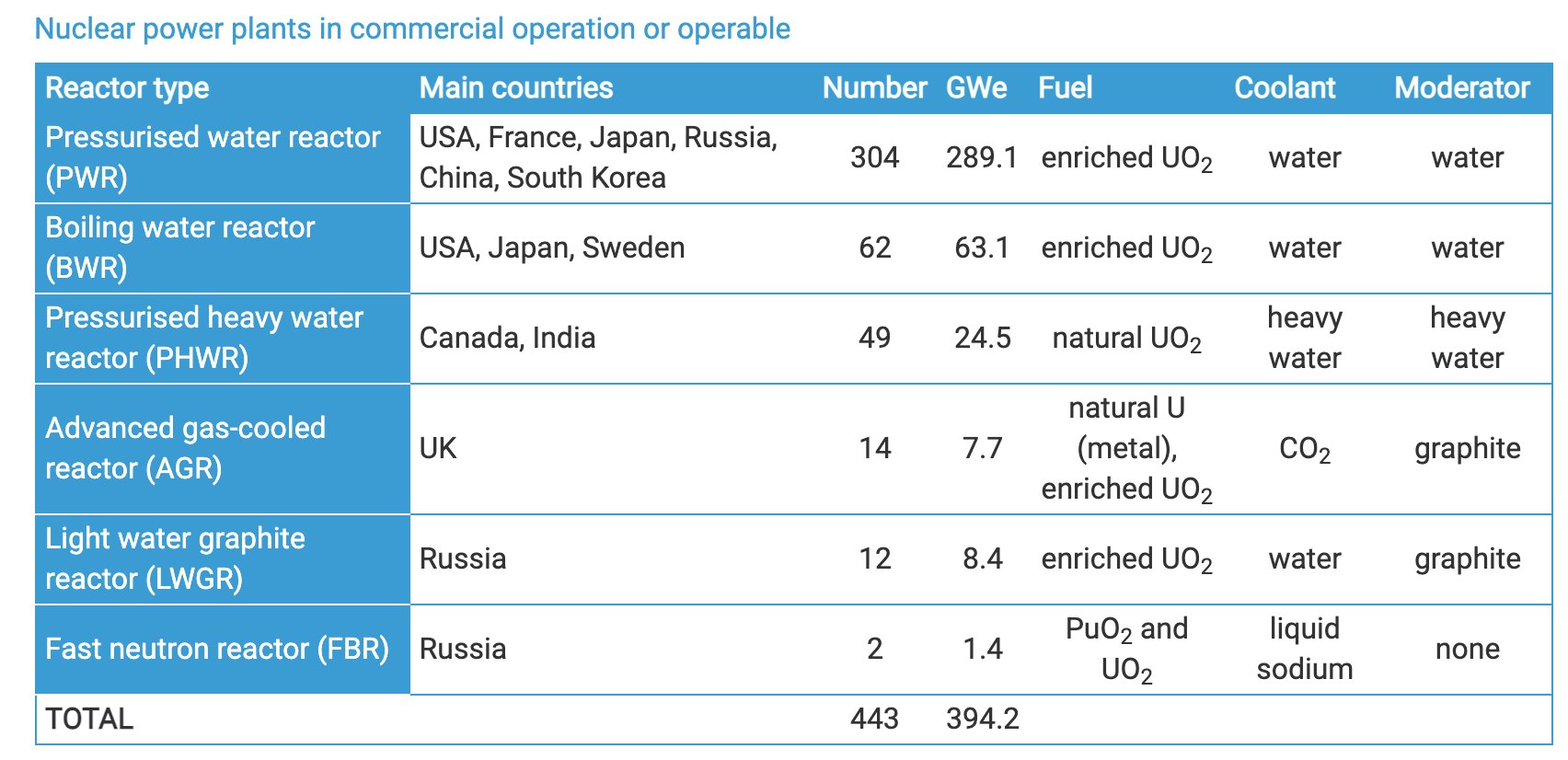
U.S. nuclear plant stats
- 20% of U.S. electricity from nuclear
- First plant in U.S. in 1958
- 2021 stats
- 94 reactors, ~1 GW each (avg)
- 56 plants
- 28 states
- Average age is 39 years
- Oldest plant is Nine Mile Point (NY), Dec. 1969, 52 years
- Newest is Vogtle Unit 4, 2023, Georgia

Shippingport Power Station, Pennsylvania
U.S. nuclear plants timeline
World nuclear plants
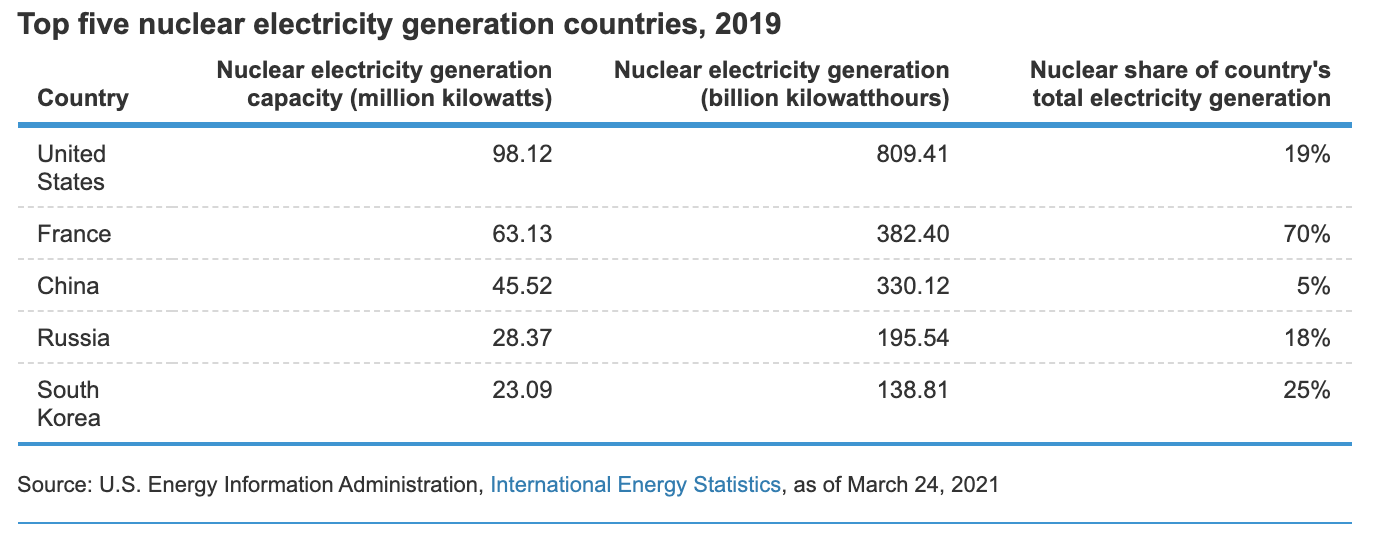
- ~156 plants in service (> 1000 MW)
- Google “list of nuclear reactors” –> people also ask…
World nuclear plants
Exercise: World Electricity as Nuclear
- Look up the world total electricity production. Energy Institute
- Convert this to CMO
- If run continuosly over the year, find the power in GW
- What is the largest nuclear power plant, and it’s power?
- What is the average size nuclear power plant > 1000 MW?
- How many of the largest and average size plants are needed?
- If one plant is built per week, how many years are needed?

Exercise: World Electricity as Nuclear
- BP: 26823 TWh \(\cdot\) 3600 s/h = 9.65628E19 J (per year)
- 1.6E20 J/CMO \(\rightarrow\) 0.6 CMO
- 9.65628E19 J/yr \(\cdot\) yr/(3600 \(\cdot\) 24 \(\cdot\) 365 s) \(\cdot\) GW/1E9 W = 3062 GW
- Largest plant is the Kashiwazaki-Kariwa
(K-K) Plant in Japan
- 7965 MW
- Avg size plant = 2382 MW (for plants over 1000 MW)
- 155 plants over 1000 MW
- # K-K plants = 3062/7.965/0.92 = 418
- with a 92% capacity factor
- 1 plant per week for 8 years
- # avg plants = 3062/2.382/0.92 = 1397
- 1 plant per week for 27 years
Isotope binding energy
Isotope Binding Energy

Stardust
Genesis 3:19 …for dust thou art, and unto dust shalt thou return
Emission/Decay
- \(\alpha\) (helium nucleus), e.g.,
- \(^{238}_{92}\text{U} \rightarrow ^{234}_{90}\!\!\text{Th} + ^4_2\!\text{He}\)
- \(\beta^-\) (emitted electron)
- \(n \rightarrow p + e^- + v_e\)
- \(\beta^+\) (positron, ~ a
positively charged electron)
- \(p \rightarrow n + e^+ + v_e\)
- \(\gamma\) (high energy photon)
- often occurs from an excited daughter nucleus after \(\alpha\) or \(\beta\) decay
Table of Nuclides
Half life
Equation for radiactive decay?
- Radioactive decay is governed by \(\frac{dN}{dt} = -N/\tau\), \(N(0) = N_0\).
- Here, \(\tau\) is the characteristic decay time.
- The analytic solution is
\[N = N_0e^{-t/\tau}\]
- Relate this to the half-life: \(\frac{N}{N_0} = \frac{1}{2} =
e^{-t_{1/2}/\tau}\rightarrow \tau = -t_{1/2}/\ln(1/2)\) \[\tau = t_{1/2}/\ln(2)\] \[t_{1/2} = \tau\ln(2)\]
\[N = N_0\left(\frac{1}{2}\right)^{t/t_{1/2}}\]
Radiation penetration
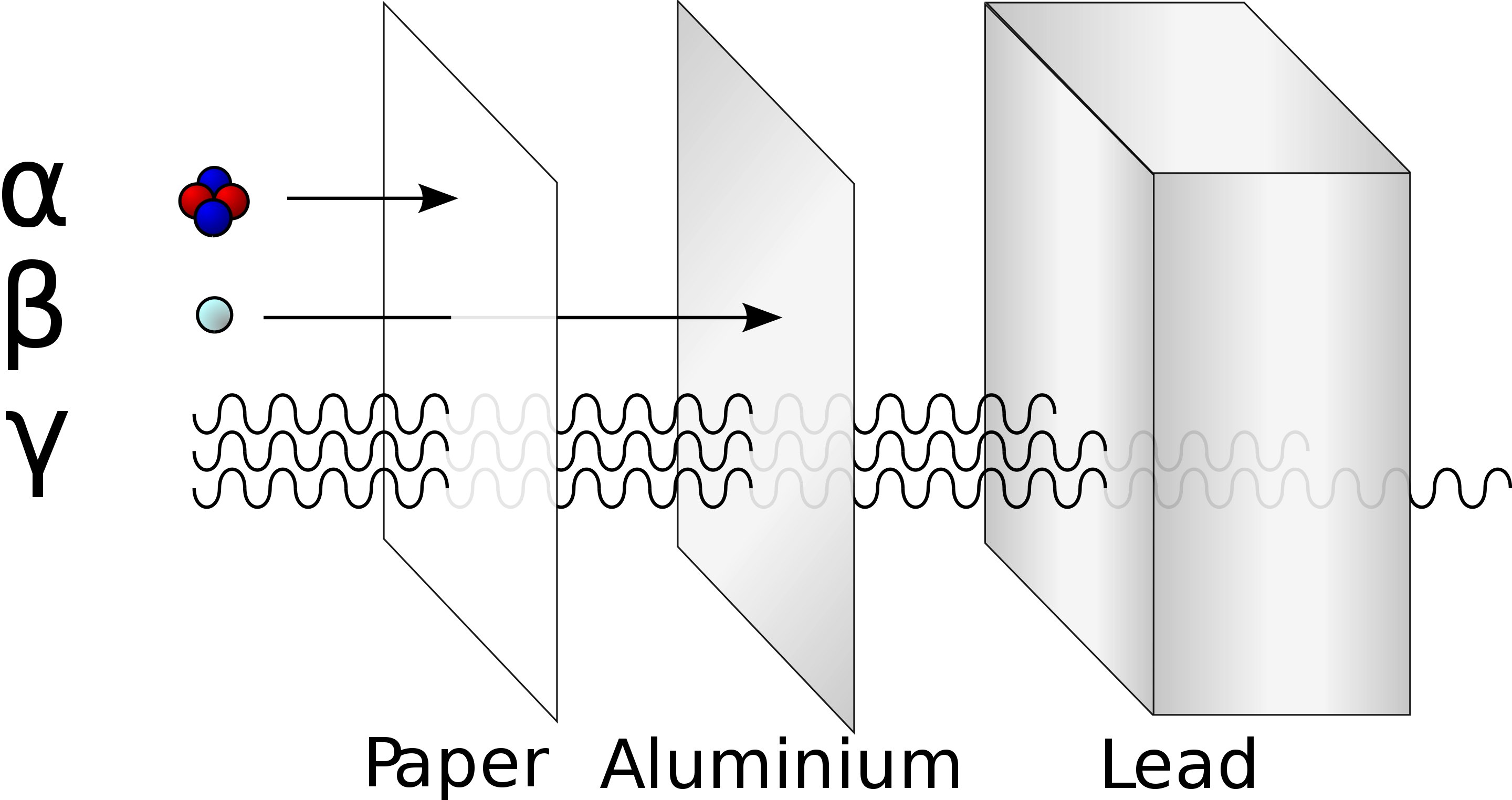
- \(\alpha\) stopped by paper, dead
outer layer of skin (dangerous if injested)
- Polonium-210 poisoning of Alexander Litvinenko
- \(\beta\) stopped by aluminum foil, can penetrate ~1/2 inch into skin
- \(\gamma\) stopped by lead, thick concrete
Radiation penetration
Ionization demonstration
U-235 Fission
- \(^{235}U + n \rightarrow [^{236}U]
\rightarrow ^{144}Ba + ^{89}Kr + 3n\)
- typical
- 1n \(\rightarrow\) 2.5n daughter neutrons
- prompt neutrons are fast:
- ~2 MeV = 19.5 million m/s (7% of light).


Chain reaction
- Neutron fate:
- Fission
- Absorbed (e.g., by U-238)
- Escape
- (bare n is unstable \(\rightarrow\) p+\(e^-\))
- half life ~ 10 minutes.
- Chain
reaction
- 1 fission creates \(\ge\) 1 fission
- Energy from mass: \[\Delta m = m_\text{reactants} -
m_\text{products}\] \[\Delta m =
\frac{E}{c^2}\]
- mass difference as kinetic energy of fission products \(\rightarrow\) slows \(\rightarrow\) heat.
- Neutrons + protons are conserved, electrons are not (formed).
- Energy released as more stable nuclei are formed (sounds familiar)

Exercise: mass \(\rightarrow\) energy
How much mass converted fully to energy equivalent to 1 CMO?
- 1 CMO = 1.6E20 J
- \(E = mc^2\) \(\rightarrow\) \(m = E/c^2\)
- c = 299792458 m/s
- m = 1780 kg
- ~ 2 m\(^3\) of oil (@ 890 kg/m\(^3\))
- Volume is 9 billion times less than 1 CMO
Of course, nuclear reactions never approach full mass
conversion.
1 CMO requires about 2000 tons of U-235 fission
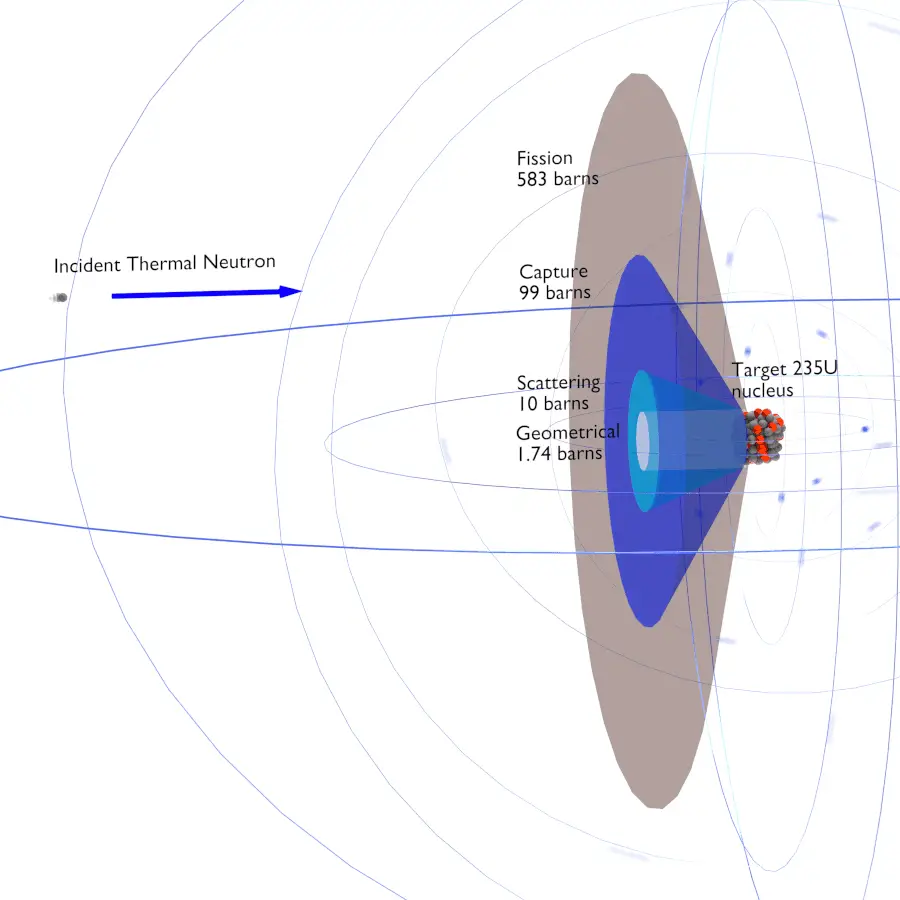
Cross section
- Cross section is an area of interaction of a target particle
- May be bigger or smaller than the geometrical area
- In this sense it’s like a probability of interation
- Units: 1 barn = 10\(^{-28}\) m\(^3\)
- as in “you couldn’t hit the broad side of a barn”
- Depends on the nucleus and the neutron energy
- Fast (prompt) neutrons are released during fusion (0.1 - 10 MeV).
- These have low fission cross sections though, so they are slowed down to “thermal” by neutron interactions with a moderator.
- Thermal neutrons (0.025 eV at 17 \(^o\)C, ~2.2 km/s=4900 mph)
Cross section
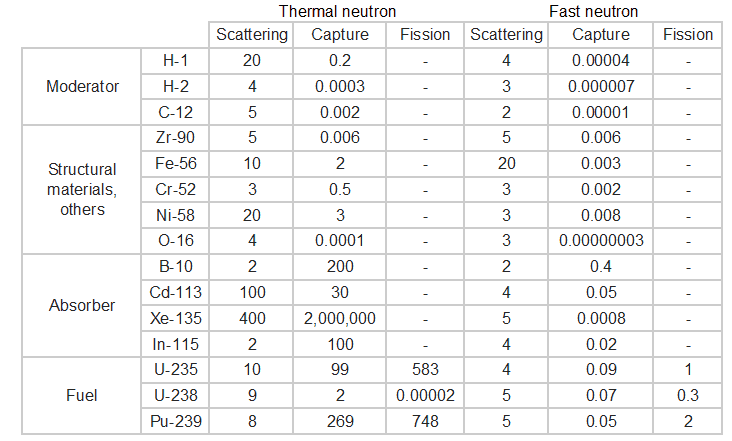
Neutron moderator
- Fast (prompt) neutrons are released during fusion (0.1 - 10 MeV).
- These have low fission cross sections though, so they are slowed down to “thermal” by neutron interactions with a moderator.
- Thermal neutrons (0.025 eV at 17 \(^o\)C, ~2.2 km/s=4900 mph)
Question: how to slow the neutrons down?
Collide with masses similar to the mass of a neutron. Recall elastic collisions. So, what to use?
Hydrogen (water)
Also consider absorption cross section.
Graphite, heavy water…
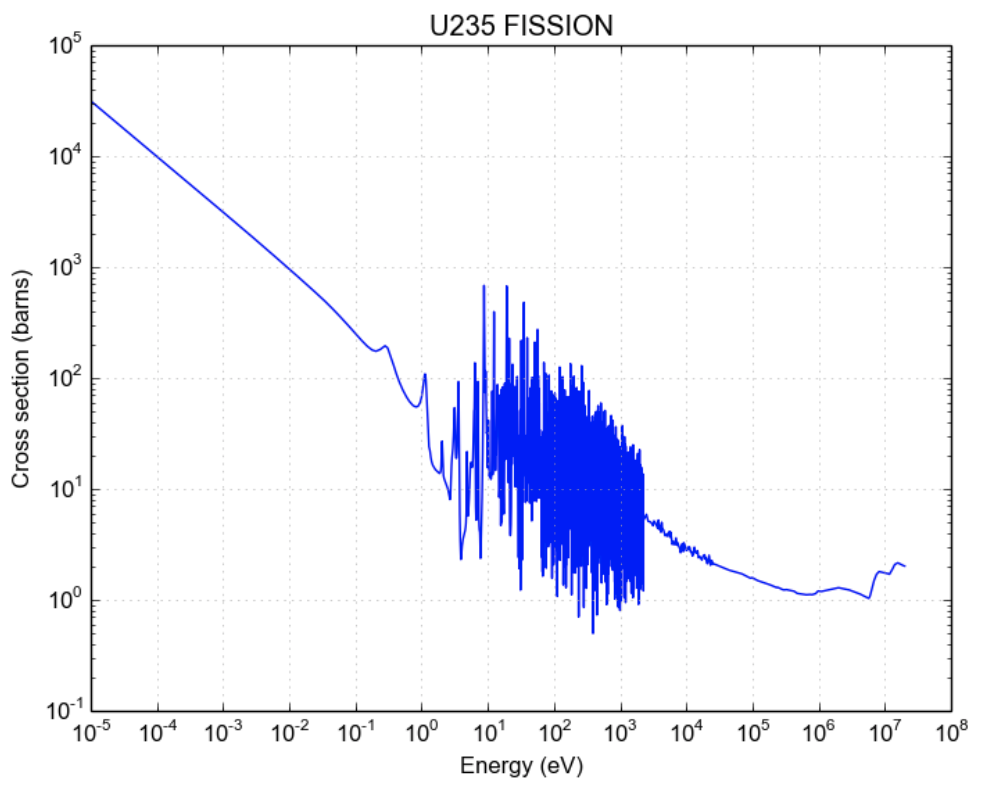
Uranium
- U-235
- 0.72% abundance
- Only natural fissile isotope
- half life of 703.8 million years
- \(\rho=19.1\) g/cm\(^3\) (lead is 11.3)
- U-238
- 99.27% abundance
- non-fissile
- half life of 4.468 billion years
- can form Pu-239


Uranium deposits
- 40 times more common than silver, 500 times more common than gold.
- Found everywhere.
- Highest grade deposits in Canada

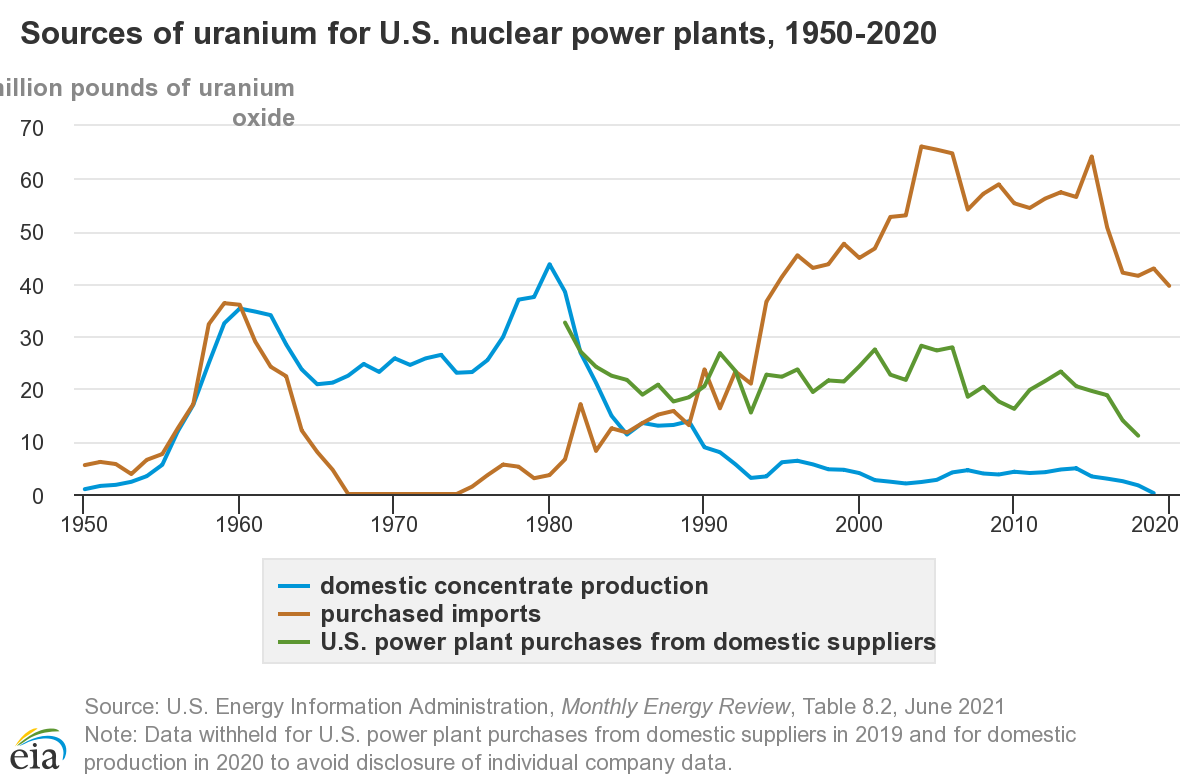
US Uranium sources
Uranium processing
- Uranium oxide, dissolved with acid, then precipitated as U\(_3\)O\(_8\) (yellowcake)
- Mined where concentration 0.5-5%
- Mining type
- underground
- open pit
- gold and phosphorus tailings.
- In-situ
leach (ISL) mining (57%)
- sulfuric acid or sodium bicarbonate
- Enrichment
- Reaction to UF\(_6\) (gas, liquid,
solid).
- reacts with water to form HF, corrosive!
- Ultracentrifuge
to separate \(^{238}UF_6\) from \(^{235}UF_6\) based on density differences.
- MW = 349, 352 (0.8% different).
- ~100,000 RPM
- Reaction to UF\(_6\) (gas, liquid,
solid).

Uranium reserves
- 8
million tonnes (2019) uranium metal
- “Reasonably assured and inferred” resource
- Recoverable at market prices 40-260 $US/kgU
- From the “red book”
- 80 years at current rate of consumption with conventional reactors
- Current reactors (in the U.S.) are “once through.”
- Reprocessing
- Gain 25-30% more energy from the original uranium.
- reduce volume of waste
- reduce level of radioactivity
- Breeder reactors
- produce more fuel than they consume
- U-238 (forming Pu-239), “fast” reactor
- Th-232 (forming U-233) “thermal” reactor
- Thorium is 3.5 times as common as uranium.
- could raise fuel availability 40-50 times
“It has been estimated that doubling the price of uranium ore, which as mentioned above would increase the cost of the electricity by less than 1 ¢/kWh, would increase the known conventional resources base 10-fold. This increase could easiliy support a much larger (CMO/yr-level) role of nuclear power for several centuries” –Crane et al., A cubic mile of oil, 2010.
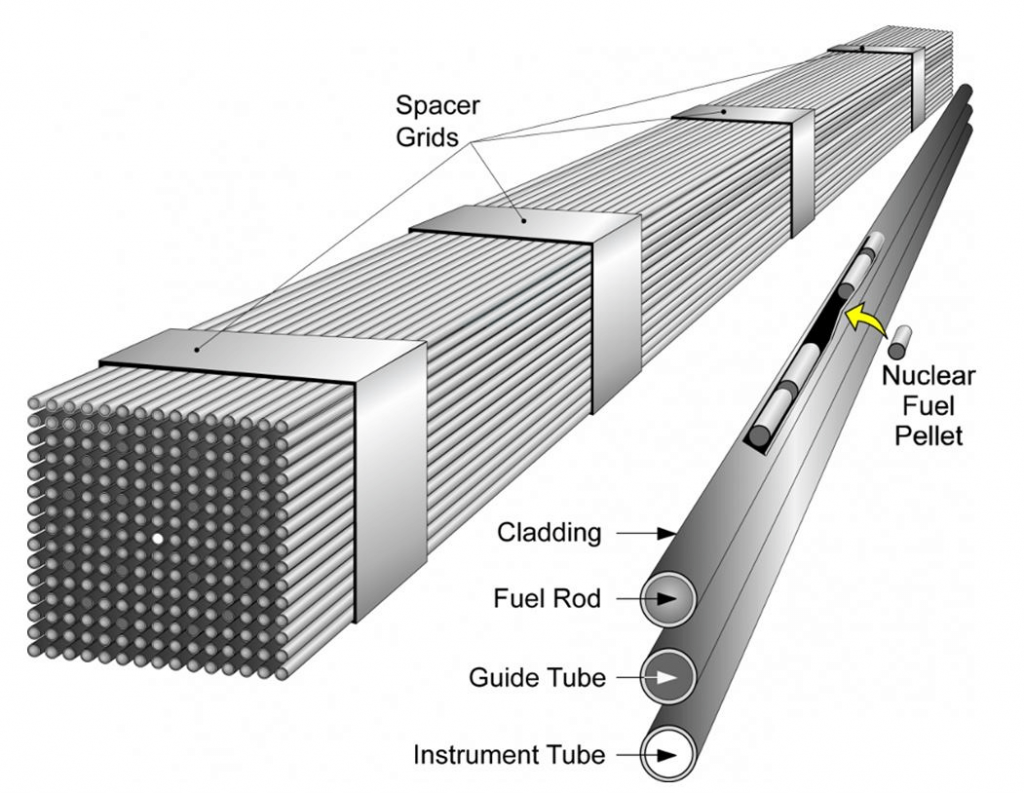
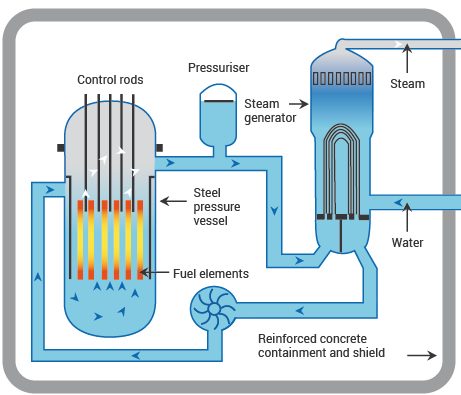
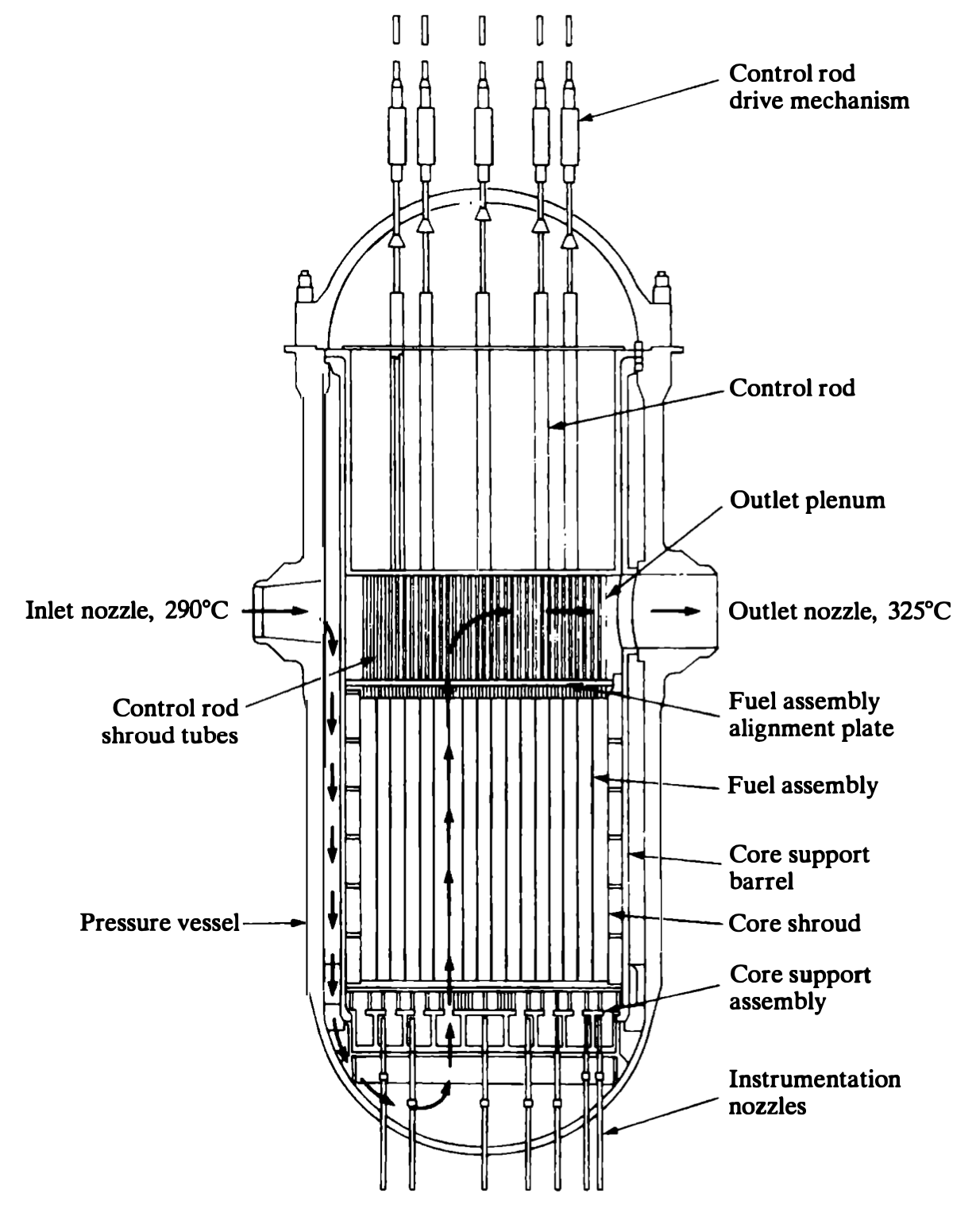
Nuclear reactors
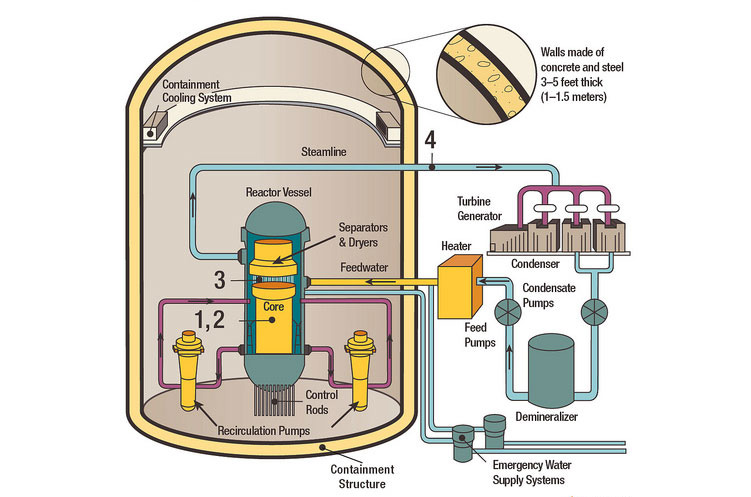
Nuclear reactors: PWR
- Pressurized water reactor
- Pressurized water in contact with fuel
- Water transfers heat and moderates the neutrons
- Nonboiling (high P)
- Heat transfer to separate steam loop
- T: 290 \(\rightarrow\) 325 \(^oC\)
- P: 15 MPa (2250 psi)
Nuclear reactors: PWR
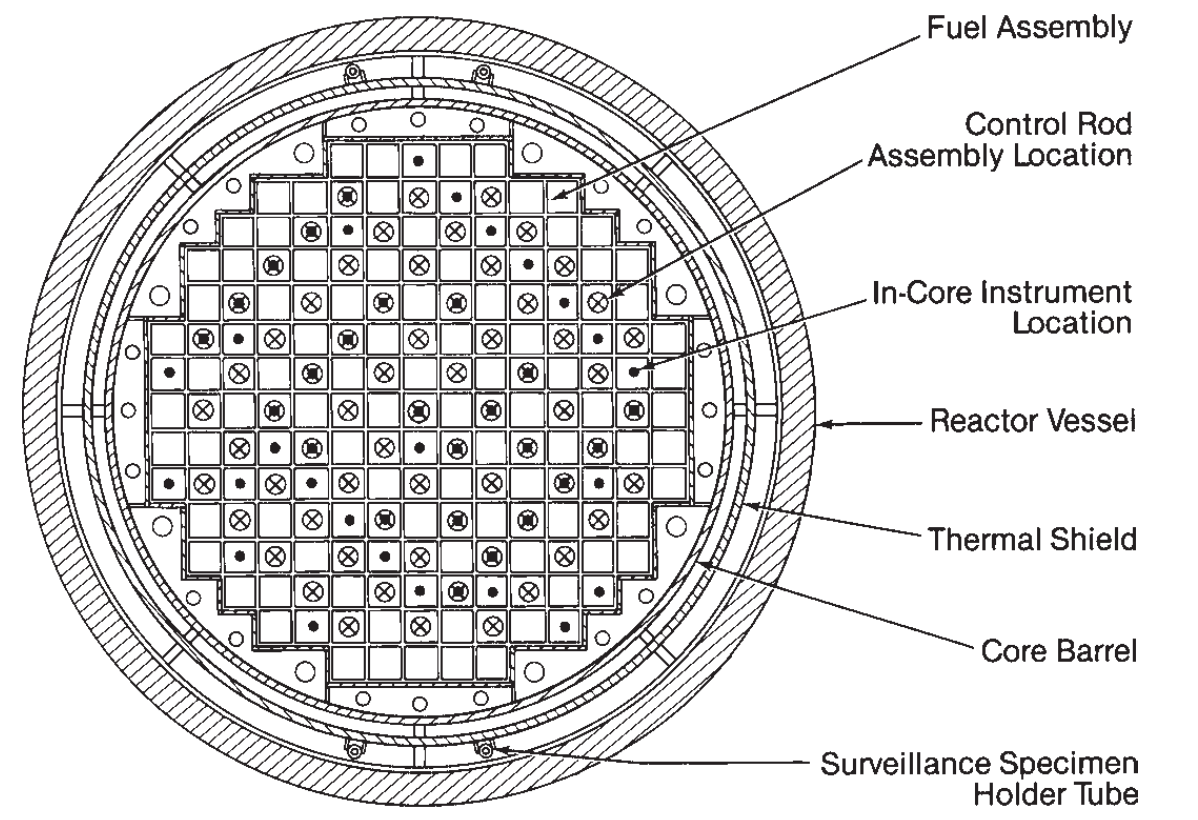
Nuclear reactors: PWR
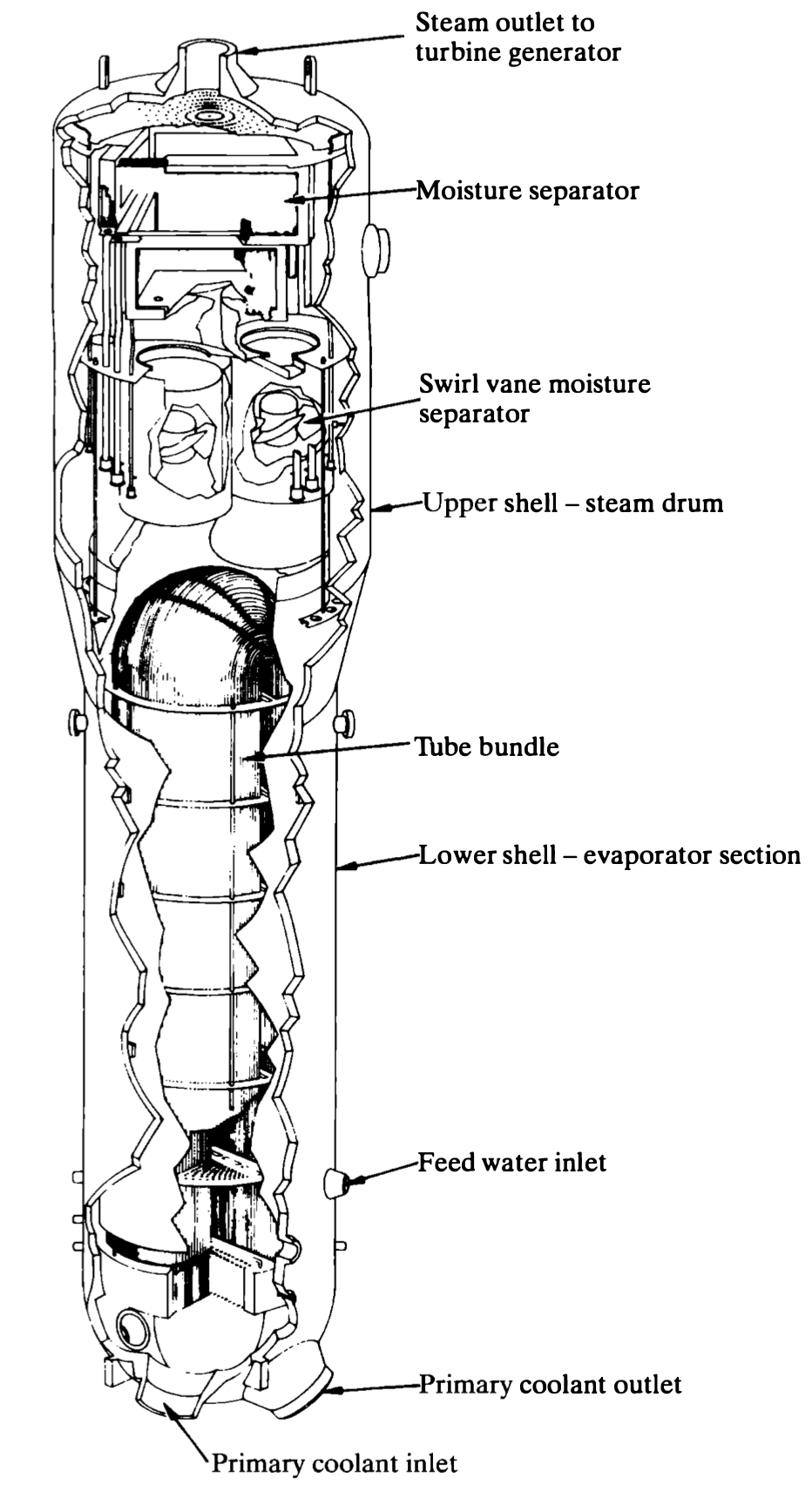
Nuclear reactors: BWR
- Boiling water reactor
- Steam is formed directly in the reactor (direct cycle)
- No separate heat transfer loop or steam generators needed.
- Latent vs sensible heat \(\rightarrow\) less water flow.
- Water becomes radioactive \(\rightarrow\) turbine, pumps, pipes, etc. shielded.
- P: 7 MPA (900 psi)
- T: 290 \(^oC\)
- Larger unit for same energy vs. PWR; (BWR lower energy per volume)
Nuclear reactors: PHWR/CANDU
- Pressurized Heavy Water Reactor/Canadian Deuterium-Uranium
- Use natural, unenriched Uranium
- Light water absorbs some neutrons requiring enriched U
- Deuterium (heavy water) doesn’t absorb thermal neutrons.
- But less efficient at moderating neutrons \(\rightarrow\) longer travel, larger vessel.
- Also, unenriched fuel has a lower fissile density \(\rightarrow\) larger vessel.
- Large pressure vessel is expensive and complex, so the overall
reactor is cool, unpressurized, filled with D\(_2\)O moderator, but penetrated by
horizontal pressure
tubes containing the fuel and coolant with a surrounding CO\(_2\) shroud.
- can be refueled during operation.
- P: 8 MPA (1285 psi)
- T: ~305 \(^oC\)
- Indirect steam cycle
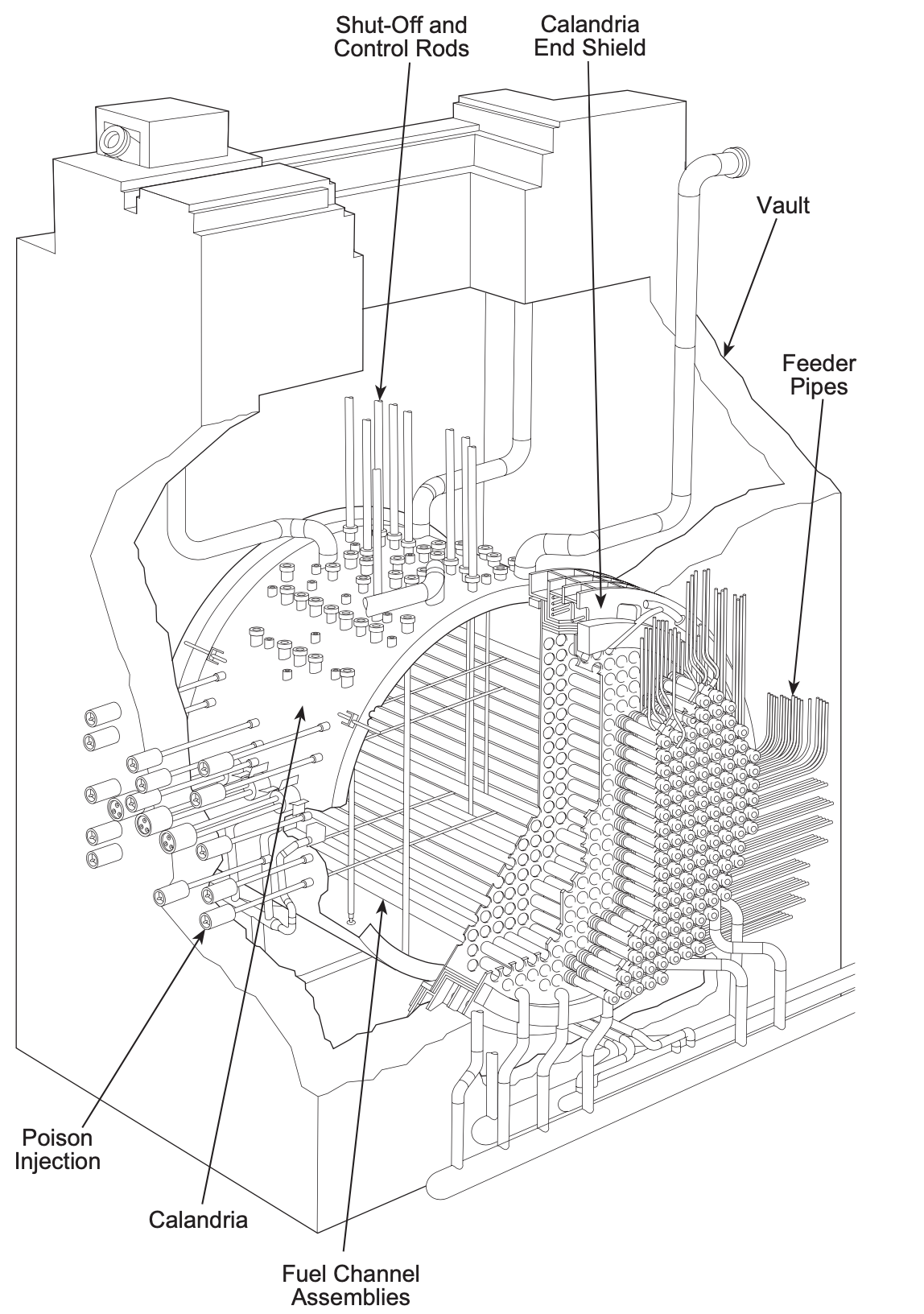
Nuclear reactors: PHWR/CANDU
Nuclear reactors: AGR
- Advanced Gas Reactor
- CO\(_2\) cooled
- non-reactive with fuel or moderator, non-absorbing
- not pressurized
- Graphite moderated
- enriched U-235 (2.5-3.5%)
- T: 650 \(o^C\).
- Higher efficiencies: 41%
- Indirect steam but heat transfer internal to the reactor vessel.

Nuclear plant tour
Economics
- Dominated by capital costs
- Fuel costs are around 15% of the cost of the power
- Compared to 30% and 70% for coal and NG (~2006)
- Time to complete
- Gas-fired: 2 years
- Coal-fired: 2-4 years
- Nuclear: 3-5 or more years
- Each nuclear plant is one-of-a-kind
- Lack of standardization, contributes to long approval process
- Prices of steel and concrete
- Headline: “Steel prices are up 219% since early 2020. What to expect
next”
- fortune.com August 16, 2021
- Headline: “Steel prices are up 219% since early 2020. What to expect
next”
Economics

“The levelized cost of energy (LCOE) is a measure of a power source that allows comparison of different methods of electricity generation on a consistent basis. The LCOE can also be regarded as the minimum constant price at which electricity must be sold in order to break even over the lifetime of the project.”
Radiation exposure: units
- Energy: electron-Volt (eV); 1 eV = 1.60218E-19 J
- Decay rate
- curie Ci; 1 Ci = 3.7E10 decays per second
- becquerel Bq; 1 Bq = 1 decay per second
- Ionization producted: roentgen R
- 1 electrostatic unit (3.33E-10 coul) of charge of one sign from the interaction of \(\gamma\) radiation in 0.001293 g of air (or 1 cm\(^3\) of air at 1 atm and O \(^oC\))
- 1 R = \(2.58\times 10^{-4}\) coul/kg.
- Absorbed dose
- rad (radiation absorbed dose)
- 1 rad = 0.01 J/kg = 100 ergs/g
- gray Gy (SI unit)
- 1 Gy = 1 J/kg = 100 rads
- rad (radiation absorbed dose)
- Equivalent dose
Radiation quality factors
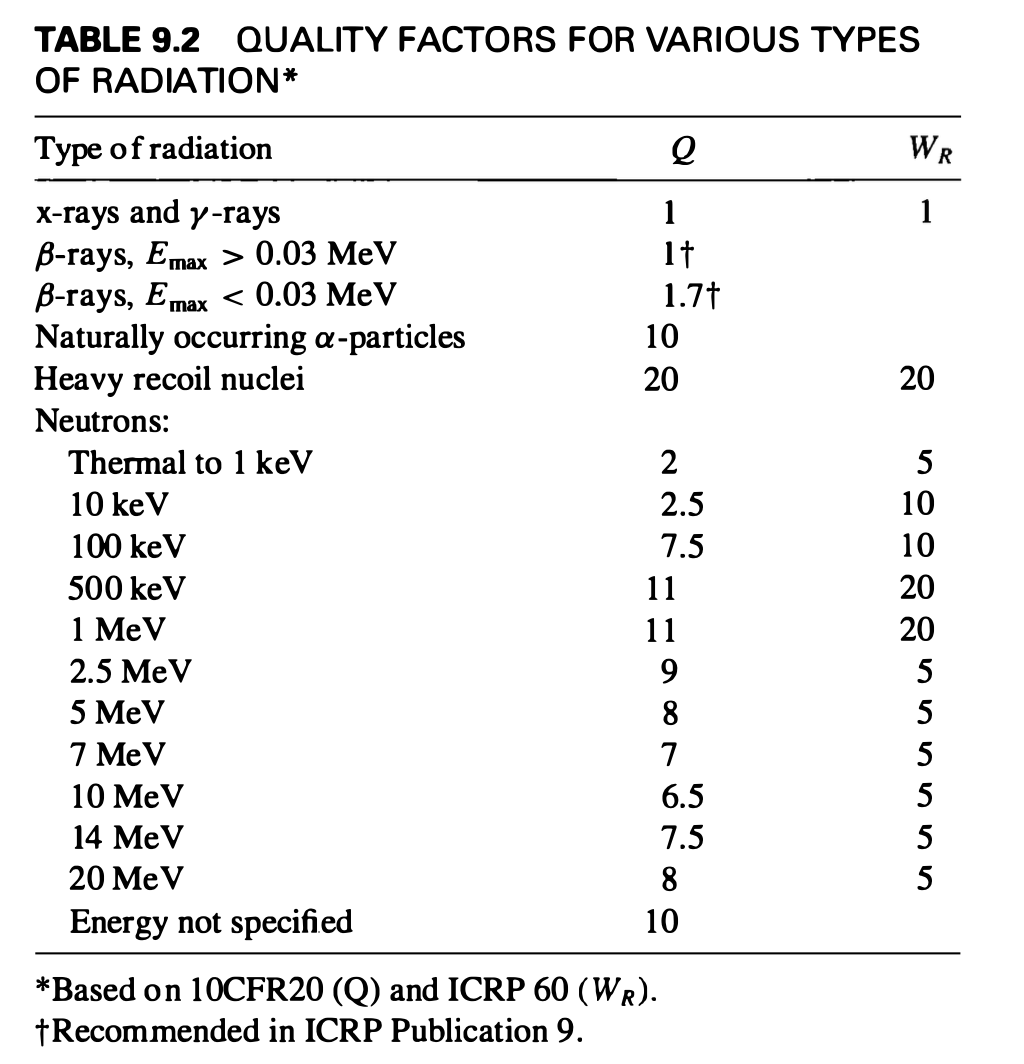
$\(Sv = Gy \cdot W_R\)
