ChEn 433 Combustion
David Lignell
Class 9-10
Nonpremixed flames
Most common combustion mode
Fuel and oxidizer are separated, mix by molecular diffusion, often aided by turbulence
Examples?
Diesel engine
Fire
Flares
Candle flame
:max_bytes(150000):strip_icc():format(webp)/178789772-56a131315f9b58b7d0bceb7e.jpg)
Demo
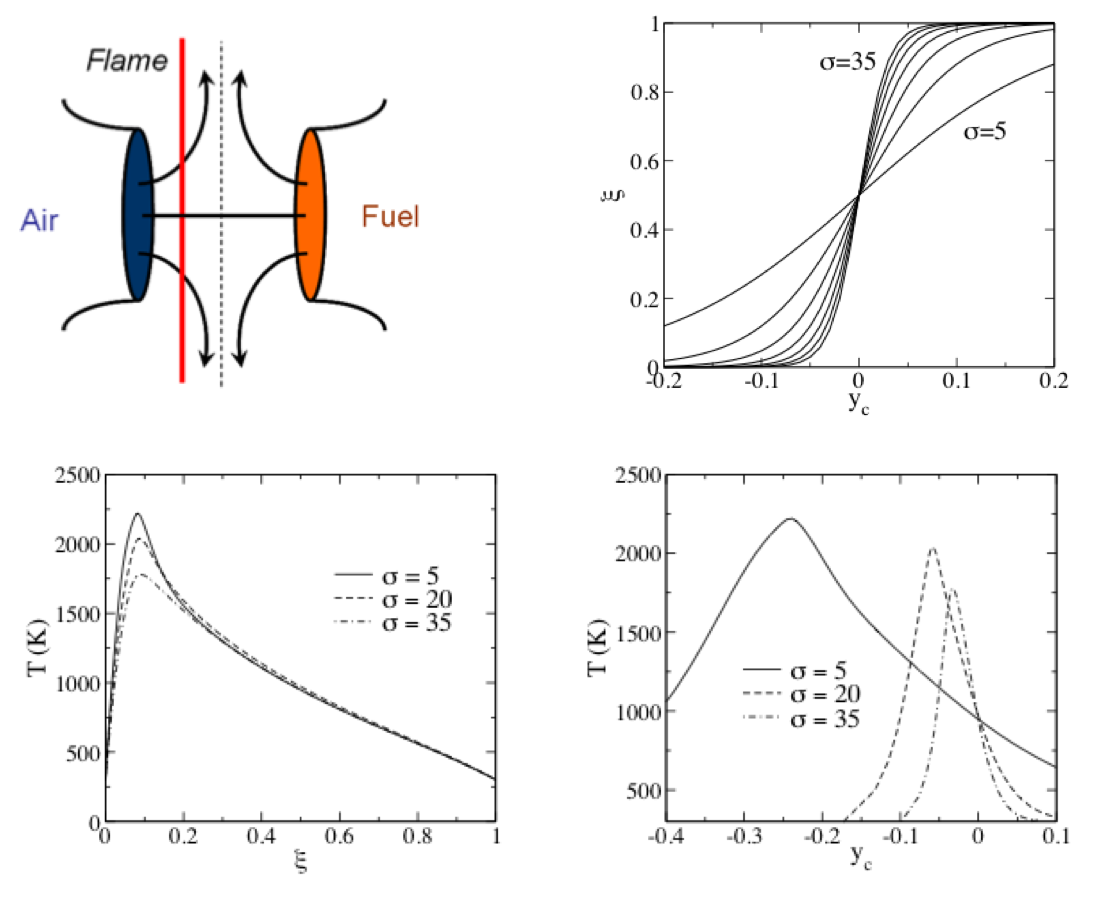
Diffusion flame structure
Diffusion flame structure
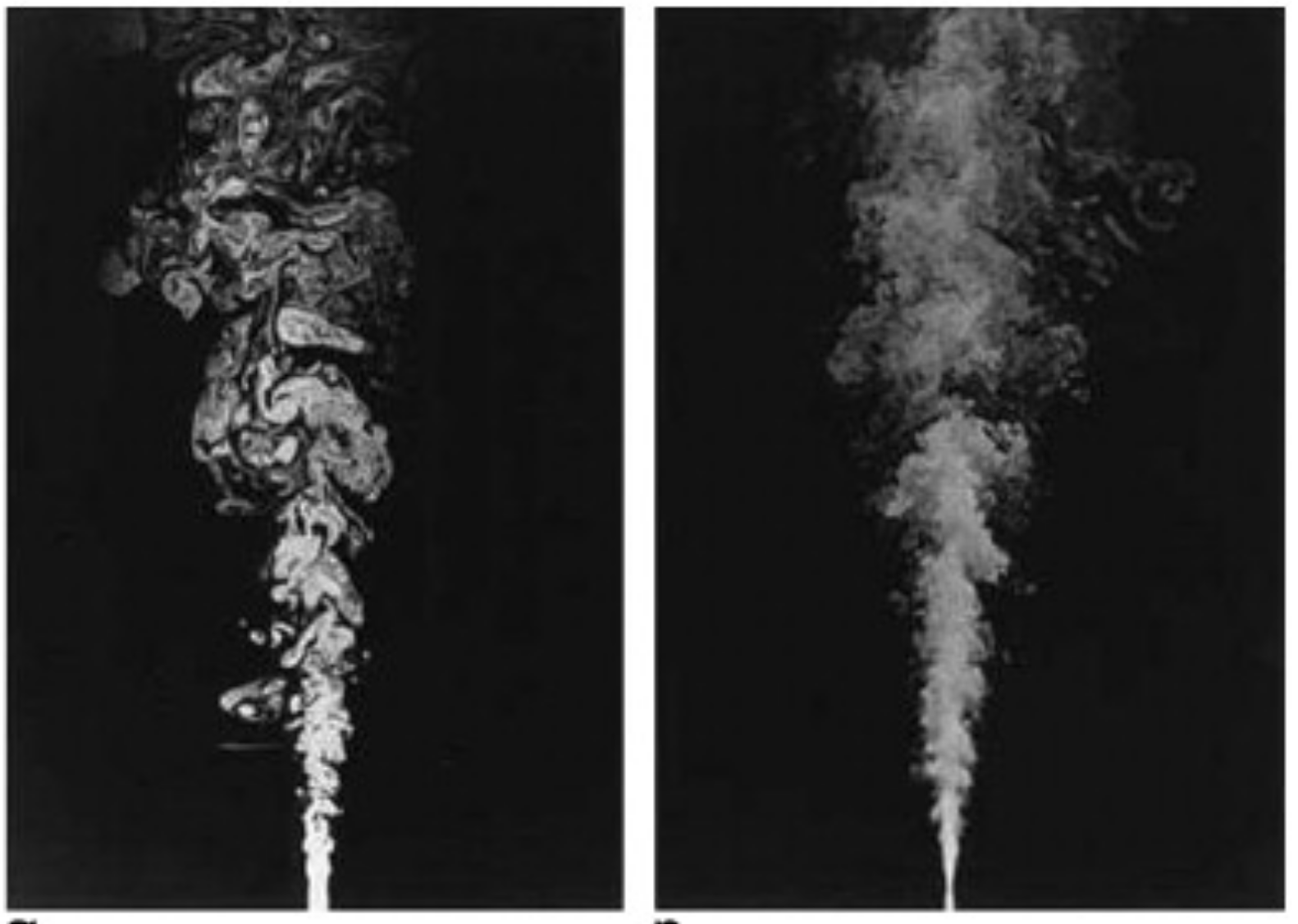
Laminar to turbulent
Turbulent swirled flame
Turbulence

Siphonaptera
Big whorls have little whorls
that feed on their velocity,
and little whorls have lesser whorls
and so on to viscosity.
—Lewis Richardson
Big fleas have little fleas upon their backs to bite ’em,
And little fleas have lesser fleas, and so, ad infinitum.
And the great fleas, themselves, in turn, have greater fleas to go on;
While these again have greater still, and greater still, and so on.
—Augustus De Morgan (mathematician, 1915)
Eternal principles
And therefore, he that will harden his heart, the same receiveth the lesser portion of the word; and he that will not harden his heart, to him is given the greater portion of the word, until it is given unto him to know the mysteries of God until he know them in full. —Alma 12:10
The works of God continue,
And worlds and lives abound;
Improvement and progression
Have one eternal round.
There is no end to glory;
There is no end to love;
There is no end to being;
There is no death above.
—W.W. Phelps, If you could Hie to Kolob
Turbulence scales
- Integral scale L
- Kolmogorov scale \(\eta\)
- Turbulent kinetic energy dissipation rate: \[\epsilon = u^2/\tau = u^3/L\]
- At high Re, small scales depend on \(\epsilon\) and \(\nu\) \[\eta = (\nu^3/\epsilon)^{1/4},\,\,\,\tau_\eta=(\nu/\epsilon)^{1/2}\] Then, with \(Re = uL/\nu\), we can obtain \[L/\eta = Re^{3/4},\,\,\,\tau/\tau_\eta = Re^{1/2}\]
Flame length
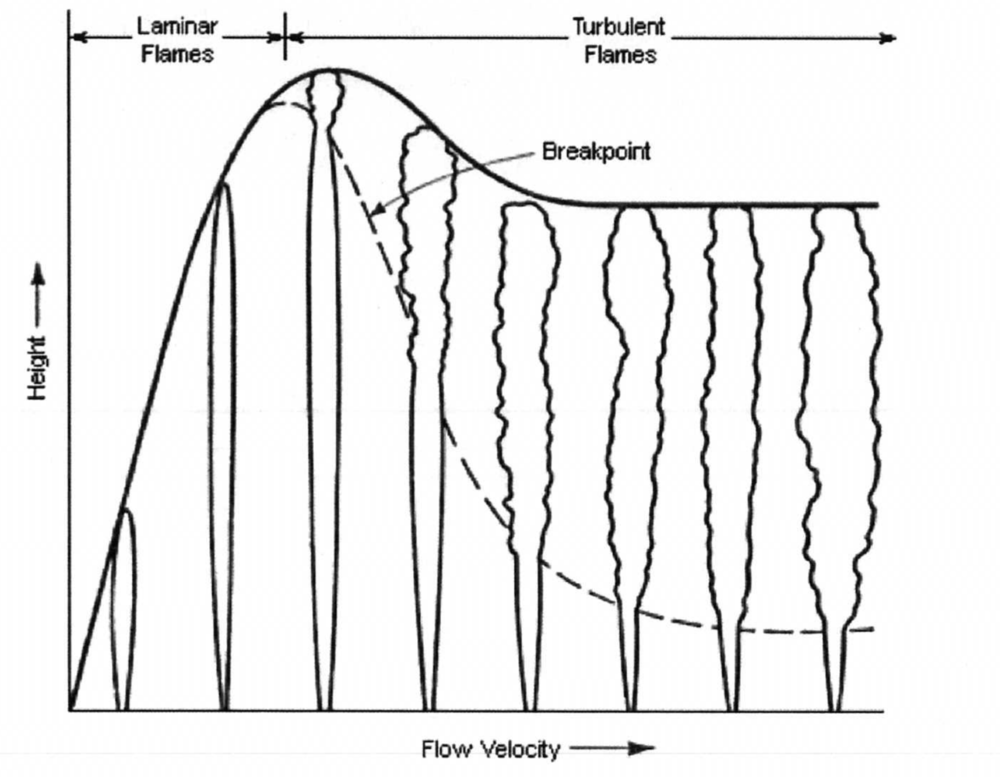
Flame length
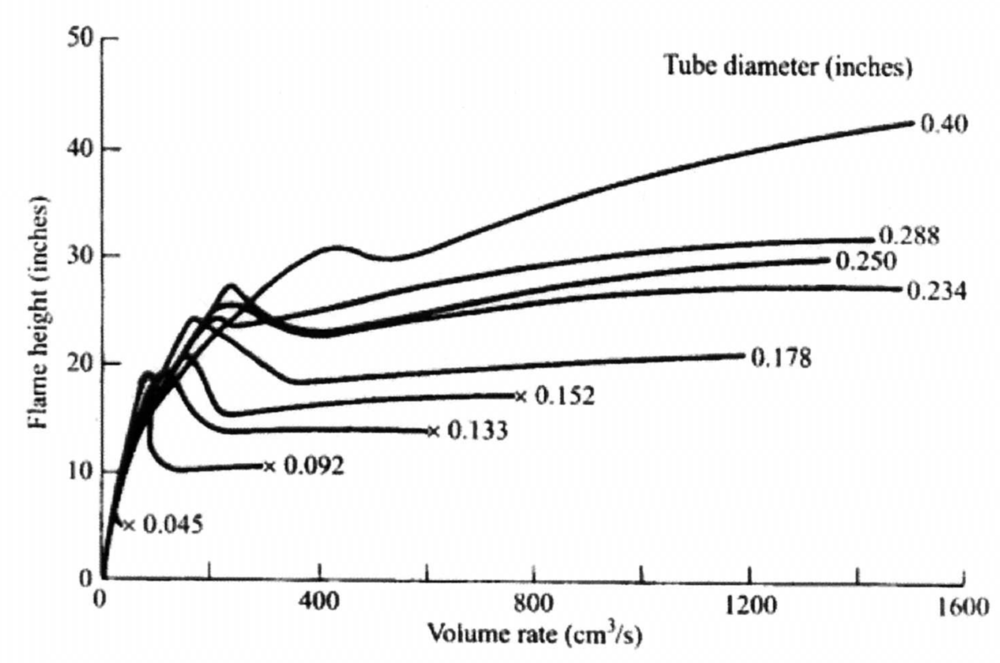
Flame length correlations
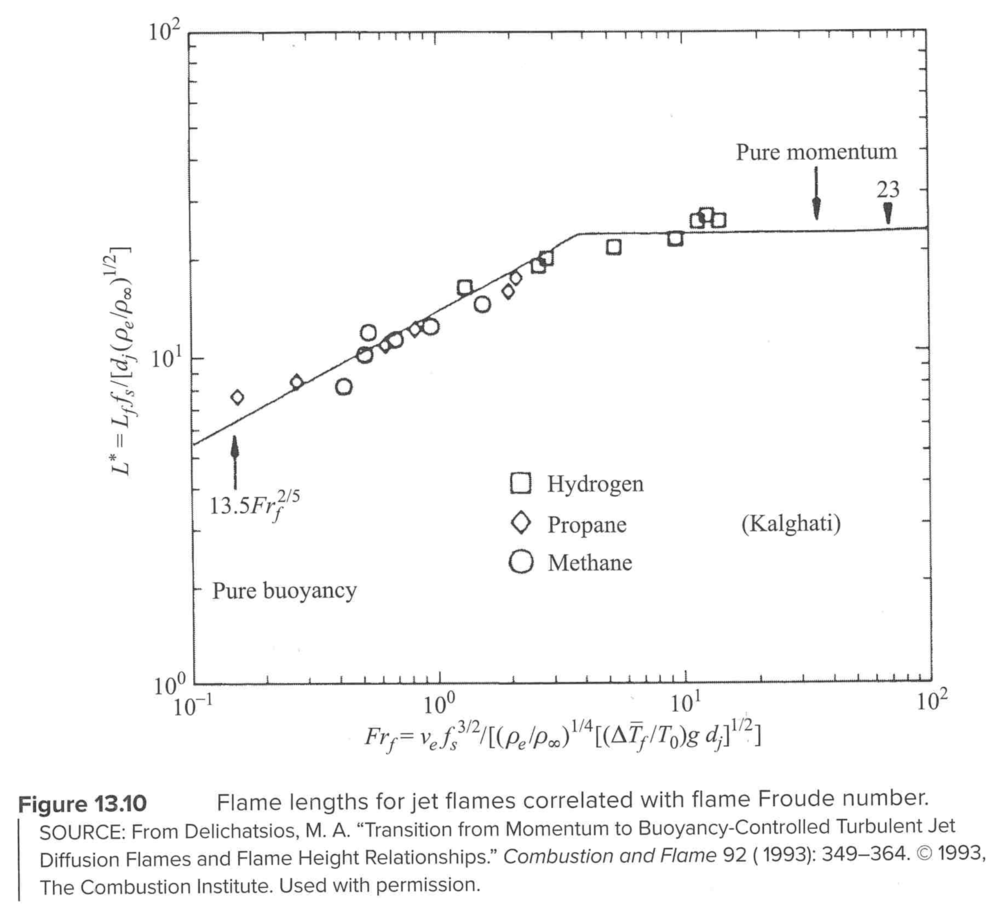
- \(v_e\)=jet exit velocity
- \(f_s\)=stoichiometric mixture fraction
- \(d_j\)=jet exit diameter
- \(\rho_e\), \(\rho_\infty\) = density at the jet exit, and in the surroundings
- \(T_0\) is really \(T_\infty\), is the surrounding temperature
- \(\Delta T_f = T_f-T_\infty\)
Note the collapse of several fuels
Soot


Soot
- Soot forms in nonpremixed and rich premixed flames, but is most important in nonpremixed flames.
- Soot is a small carbonacious particulate species
- Soot forms on the rich side of nonpremixed flames as gaseous fuels are pyrolysed.
- Soot particles consist of agglomerates of nominally round primary particles.
- Primary particle sizes are around 50 nm.
- Soot volume fractions are around 1-2 ppmv (up to 10-20).
- Soot forms at temperatures between 1300 and 1600 K.
- Formation mechanisms are highly complex and an important area of current research.
- Concentrations increase with pressure (engines)
Soot formation

- Soot forms as a progression:
- Small molecules \(\rightarrow\) ring structures
- These rings grow by acetelyne addition to form sheets
- These sheets bend and collide to form primary particles
- Primary particles agglomorate
- Particle oxidation may or may not occur
Smoke point
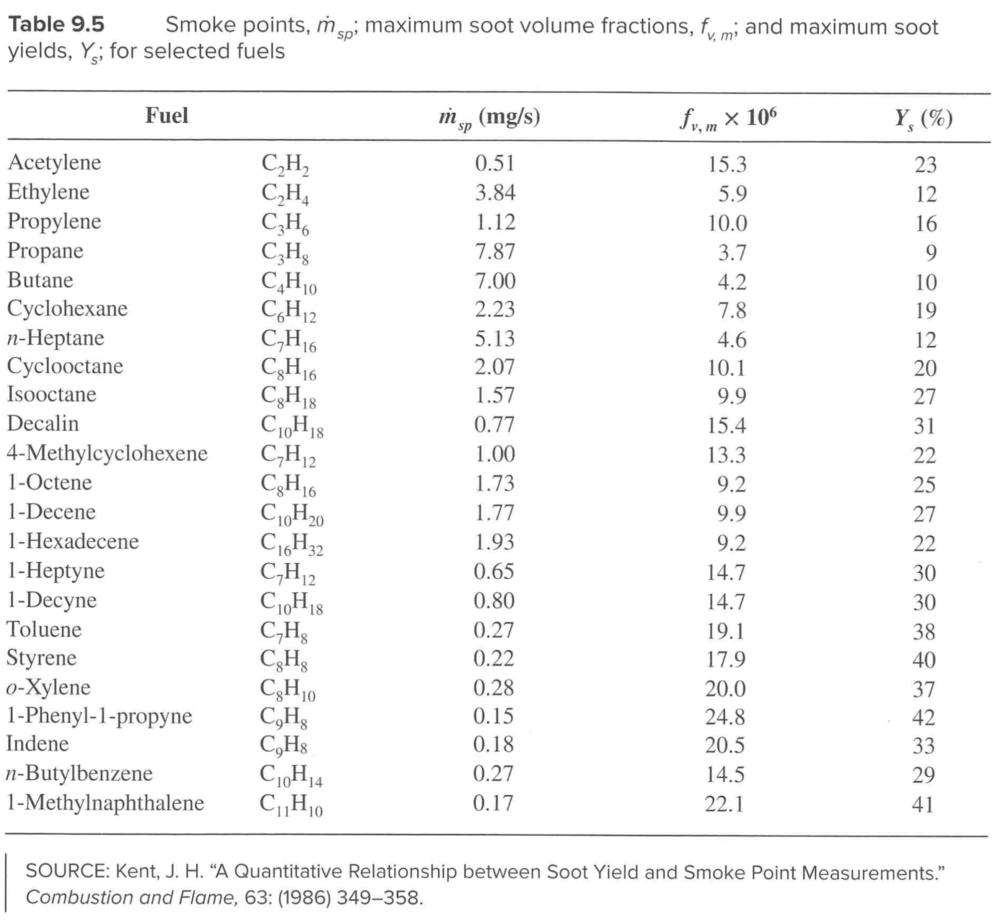
Soot modeling
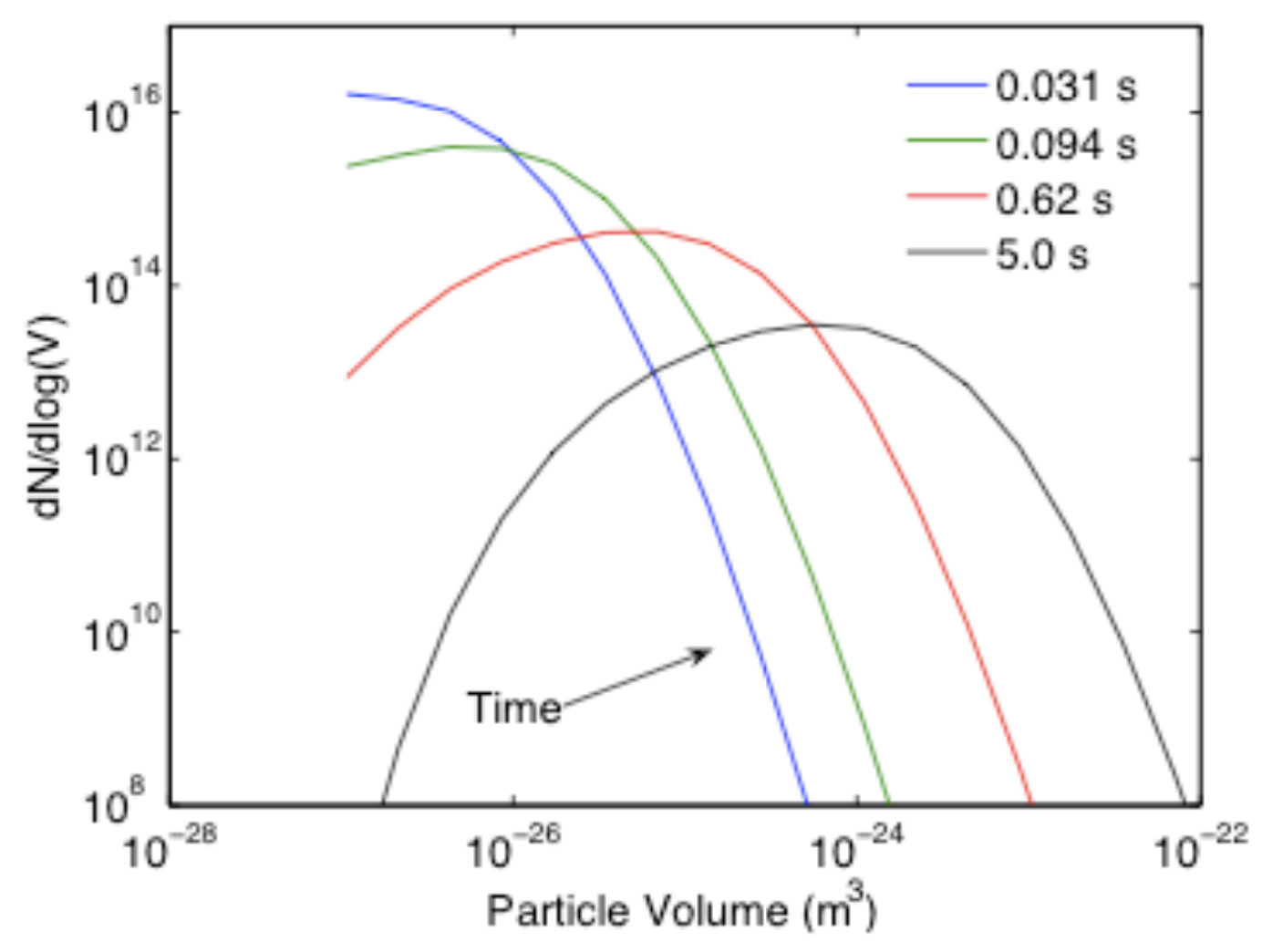
- Particle size distribution
- Direct or MC
- Sectional
- Method of Moments: \(M_0=N_s\), \(M_1=\rho Y_s\)
- Chemistry
- Empirical
- Semi-empirical (nucleation, growth, oxidation, coagulation)
- Detailed (HACA)
- Transport
- Thermophoresis: \(j_s = 0.556\nu M_r\nabla T/T\)