ChEn 433 Steam
David Lignell
Class 7
Rankine cycle
- Key power generation cycle: coal, nuclear, natural gas, etc.
- Heat input from a heat source: combustion, nuclear, geothermal, solar.
- Heat rejection: river, ocean, lake, atmosphere (dry cooling).
- Water requirements of power generation are substantial.
- Review of water consumption for electricity generation
- Water is used as the working fluid.
- available
- non-toxic, non-hazardous, non-reactive
- pump liquid, phase change gives \(Q_{isoT}\)

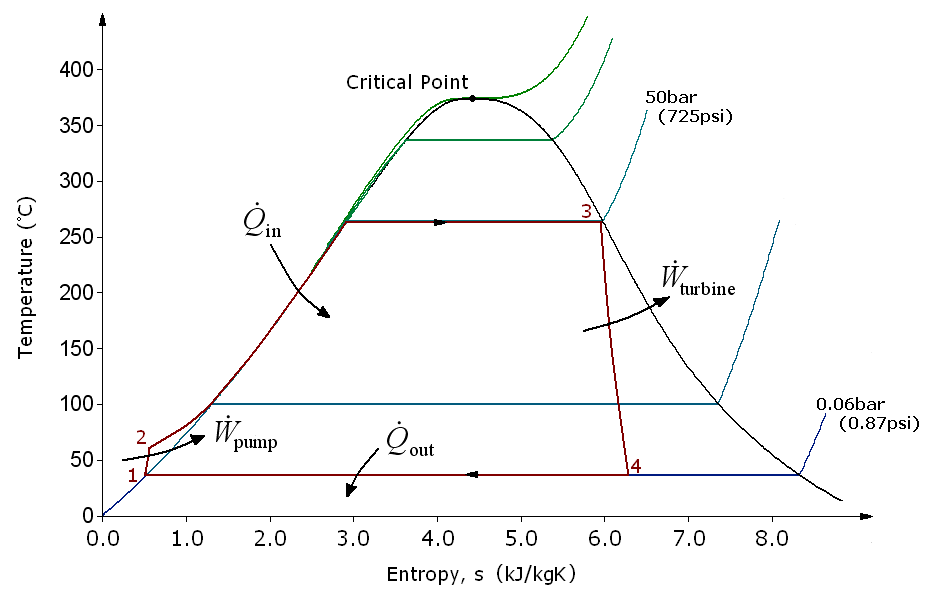
Rankine cycle
- 1-2: \(W_{in}\), pump liquid from low P to high P; isentropic compression
- 2-3: \(Q_{in}\), heat at constant pressure: boiling
- 3-4: \(W_{out}\), expand fluid through turbine; isentropic expansion
- 4-1: \(Q_{out}\), cool in condenser; vapor to liquid
Rankine cycle: issues

Issues?
- Peak temperature \(→\) efficiency \(→\) superheat
- Turbine steam quality > 90%
- Pumping a liquid vs vapor vs mixture.
- Fluid
choice
- Temperature range. Organic Rankine cycle (wiki)
- Steeper saturated vapor line.
Rankine cycle: TS
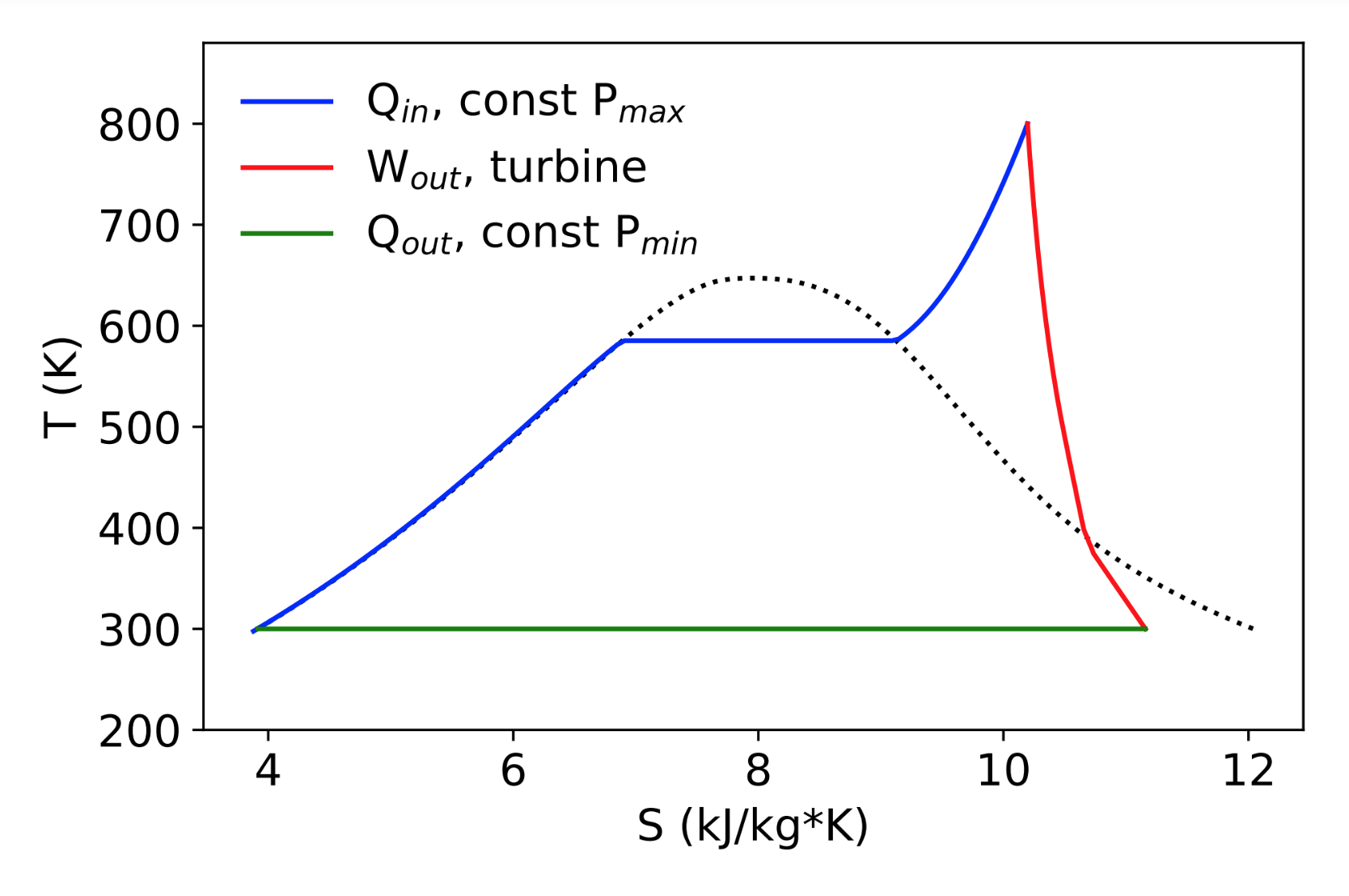
Rankine cycle: PV
What does the PV diagram look like?
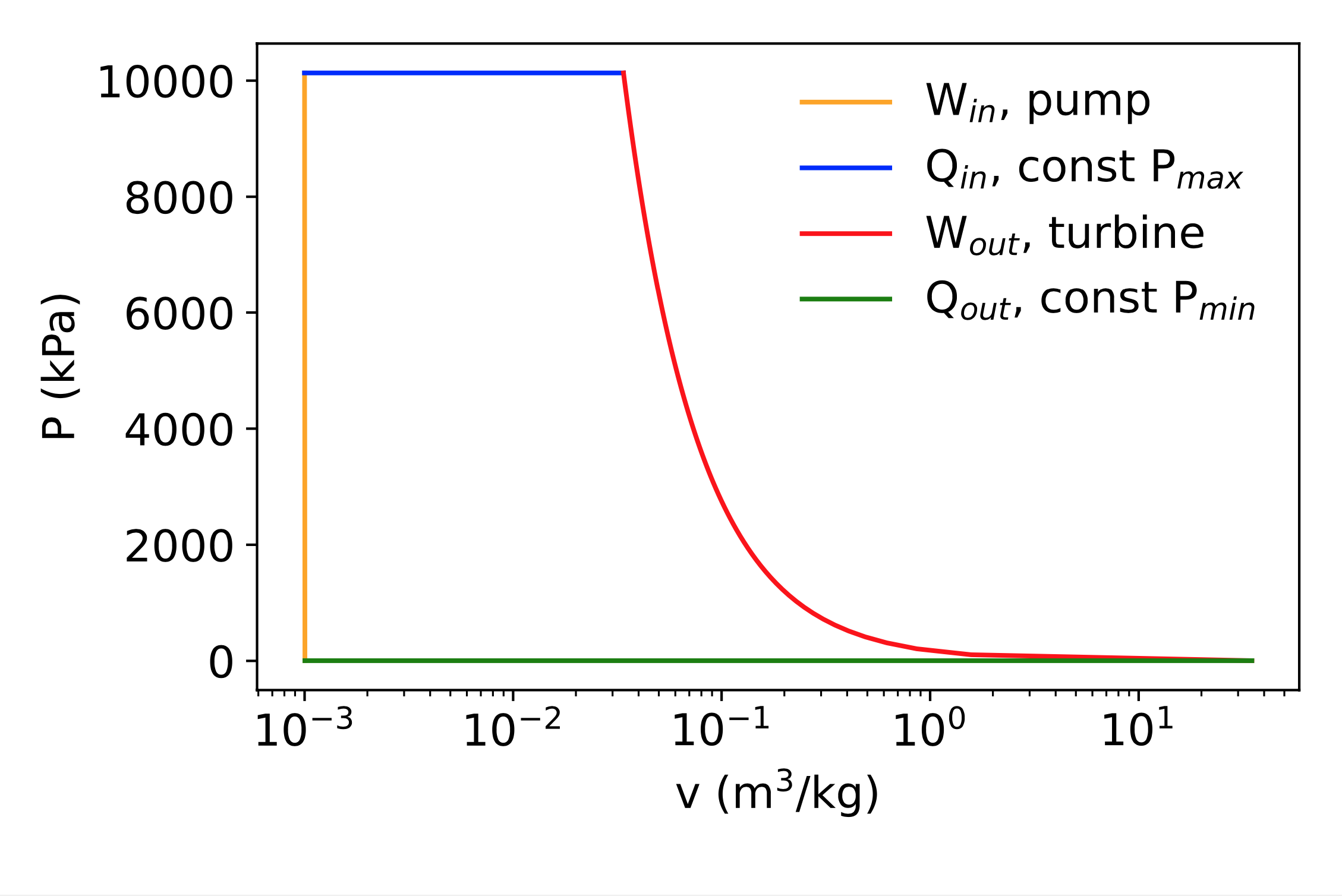
Rankine cycle: nonidealities

Nonidealities?
- Pressure drop in the boiler
- Nonideal turbine
- Subcool the condensed liquid to avoid pump cavitation
- Heat losses to surroundings
Rankine cycle: increase \(η\)

Increase efficiency?
- Decrease condenser pressure (hence temperature)
- real condensers operate below atmospheric pressure
- limited by the saturation pressure corresponding to
\(T_{cooling}\).
- river at 20 \(^oC\) \(→\) P=2312 Pa = 0.023 atm.
- Typically 2-7 kPa
- but…quality of steam in turbine, and… air leakage
- Superheat steam to higher T, even supercritical
- higher turbine exit steam quality
- but…limited by material performance and cost: 620 \(^oC\) max.
Rankine cycle: increase \(η\)

Increase efficiency?
- Increase boiler pressure (\(P_c\)=218 atm)
- Supercritical steam
- Turk Plant P > 310 bar, T > 600 \(^o\)C.
- At a given \(T_{turbine}\), higher P results in lower steam quality, so reheat
- Reheat
- 4-5% higher efficiency.
- Usually limited to single reheat stage (except for
supercritical plants)
- A second reheat gives \(Δ η\) about half the initial gain.
- Avoid superheated turbine exhaust.
- Optimal reheat pressure ~ 1/4 \(P_{max}\)
Rankine cycle: steam with reheat
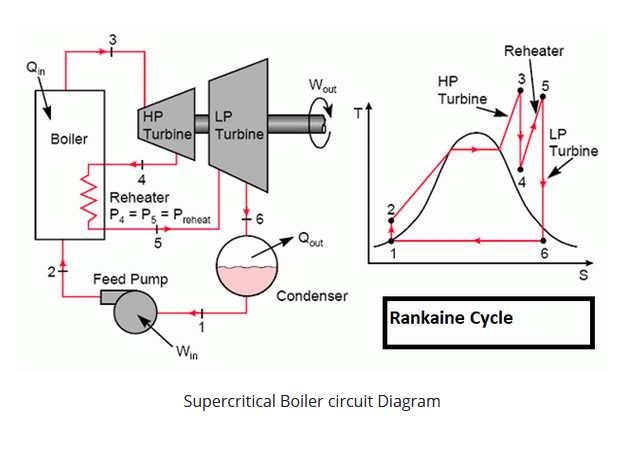
- 1-2: \(W_{in}\), pump liquid from low P to high P; isentropic compression
- 2-3: \(Q_{in}\), heat at constant pressure: heat, boil, superheat
- 3-4: \(W_{out}\), expand fluid through turbine; isentropic expansion
- 4-5: \(Q_{in}\), re-heat at constant pressure: superheat
- 5-6: \(W_{out}\), expand fluid through turbine; isentropic expansion
- 6-1: \(Q_{out}\), cool in condenser; vapor to liquid
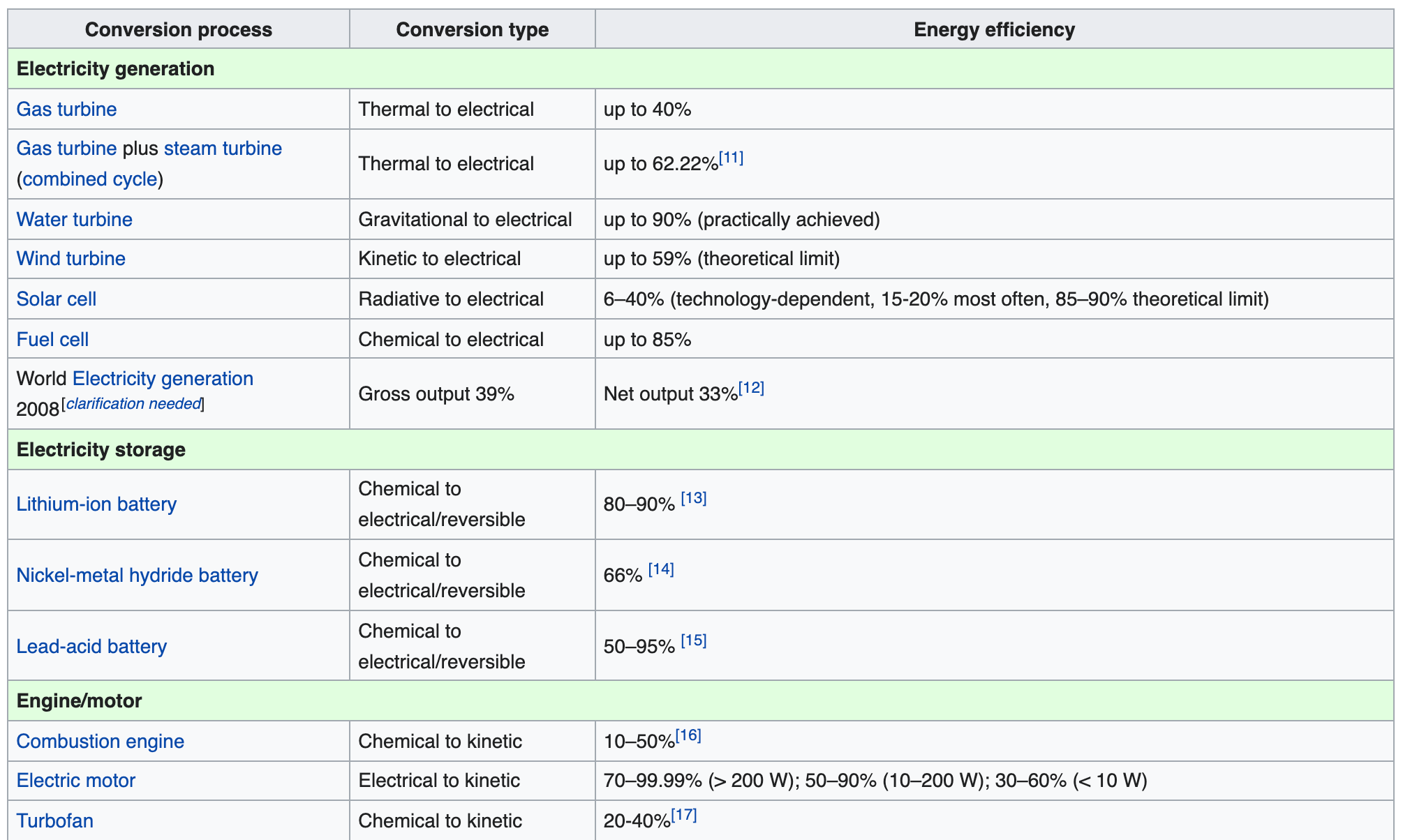
Conversion efficiencies
Steam properties: Cantera
Cantera Links
- Cantera.org
- Python thermodynamic properties
- Phase objects: water, R134a, heptane, etc.
- C++
reference
- useful for detailed documentation, units, available properties.
- General documentation
- Installation with Conda
Cantera example: steam tables
Cantera example: TS diagram
Cantera example: Rankine cycle
Work potential
- For conversion of thermal energy to electricity or mechanical work,
energy alone is not enough.
- A 1 K temperature change in the oceans would supply 17000 CMO!
- But at the average surface temperature, the thermal conversion efficiency would be ~0.003.
- We really want the work potential of an energy source.
- Names: exergy, availability, lost work, second law analysis
How much work can be extracted in going from one state to another?
Or, how much work is required in going from one state to another?
Work Potential: KE, PE
The wind blows at 10 m/s, what is the work potential per unit mass?
What is the work potential in a 5.1 meter high waterfall?
Work potential: heat transfer
We transfer 1 kW of power from a thermal energy source at 500 K.
What is the work potential?
Need to define the surrounding state, so, 300 \(K\), say.
WP here is simply 1 kW times the Carnot efficiency: \[W = 1\,\mbox{kW} ⋅ \underbrace{(1 - 300/500)}_{η_c} = 0.40\,\mbox{kW}\]
Work potential: air, change state
Air
| quantity | state 1 | state 2 |
|---|---|---|
| T (K) | 573 | 300 |
| P (atm) | 10 | 1 |
| S (J/kg\(⋅\)K) | 6892 | 6892 |
| H (kJ/kg) | 282 | 1.91 |
How much work can be extracted in moving from state 1 to state 2?
Isentropic
Work is just \(Δ H\)
That is, the maximum 1\(^{st}\)
law work can be extracted
Work potential: steam, change state
Water
| quantity | state 1 | state 2 |
|---|---|---|
| Phase | supercritical | liquid |
| T (K) | 800 | 300 |
| P (atm) | 10 | 1 |
| S (J/kg\(⋅\)K) | 11350 | 3913 |
| H (kJ/kg) | -12434 | -15858 |
How much work can be extracted in moving from state 1 to state 2?
- First law: \(Δ H = W + Q\)
- Max work for min Q
- Second law: \(Q_{min}=T_{min}Δ S\)
Lost work
- Consider an unsteady, open system
- Energy balance \[\begin{align} \frac{d(mu)_{sys}}{dt} &= \dot{W} + \dot{Q} + \sum_i\dot{m}_iu_i \\ &=\sum_i\dot{m}_iPv_i + \dot{W}_\phi + \sum_j\dot{Q}_j + \sum_i\dot{m}_iu_i \end{align}\]
Here, \(\dot{W}_\phi\) is “other” work not including injection work (which goes into enthalpy).
\[\frac{d(mu)_{sys}}{dt} = \sum_i\dot{m}_ih_i + \dot{W}_\phi + \sum_j\dot{Q}_j\]
Lost work
- Entropy balance
- Here, we define the system so that it exchanges heat with the surrounding reservoirs \(j\) at the respective reservoir temperature. In this way, all irreversibilities are within the system, and \(\dot{S}_{sys} + \dot{S}_{surr} = \dot{S}_{irrev}\). \[\frac{d(ms)_{sys}}{dt} = \sum_i\dot{m}s_i + \underbrace{\sum_j\dot{S}_{j,HT}}_{\sum_j\dot{Q}_j/T_j} + \dot{S}_{irrev}\]
\[\frac{d(ms)_{sys}}{dt} = \sum_i\dot{m}_is_i + \sum_j\dot{Q}_j/T_j + \dot{S}_{irrev}\]
Lost work
- The term \(\dot{S}_{irrev}\) is identified as the lost work divided by the surrounding temperature \(T_0\): \[\dot{S}_{irrev} = \frac{W_L}{T_0}\]
- We multiply the entropy balance by \(T_0\) and subtract the result from the energy balance. This gives a relation for the combined first and second laws: \[\frac{d(m(u-T_0s))_{sys}}{dt} = \sum_i\dot{m}_i(h_i-T_0s_i) + \sum_jQ_j\left(1-\frac{T_0}{T_j}\right) + \dot{W}_\phi -\dot{W}_L\]
- Solve for \(\dot{W}_\phi\): \[\dot{W}_\phi = \left[\frac{d(m(u-T_0s))_{sys}}{dt} - \sum_i\dot{m}_i(h_i-T_0s_i) - \sum_jQ_j\left(1-\frac{T_0}{T_j}\right)\right] + \dot{W}_L\]
Lost work
- Again: \[\dot{W}_\phi = \frac{d(m(u-T_0s))_{sys}}{dt} - \sum_i\dot{m}_i(h_i-T_0s_i) - \sum_jQ_j\left(1-\frac{T_0}{T_j}\right) + \dot{W}_L\]
- If the lost work is zero, then we have a reversible process, by which:
\[\dot{W}_{\phi,rev} = \frac{d(m(u-T_0s))_{sys}}{dt} - \sum_i\dot{m}_i(h_i-T_0s_i) - \sum_jQ_j\left(1-\frac{T_0}{T_j}\right)\]
- \(Q\) is positive into the system
- \(\dot{m}\) is positive into the system
- \(\dot{W}_{\phi,rev}\) is positive into the system