ChEn 433 Thermodynamic Cycles
David Lignell
Class 6
Outline
- Cycles
- Carnot
- Otto
- Diesel
- Brayton
- Conversion efficiencies
Carnot Cycle
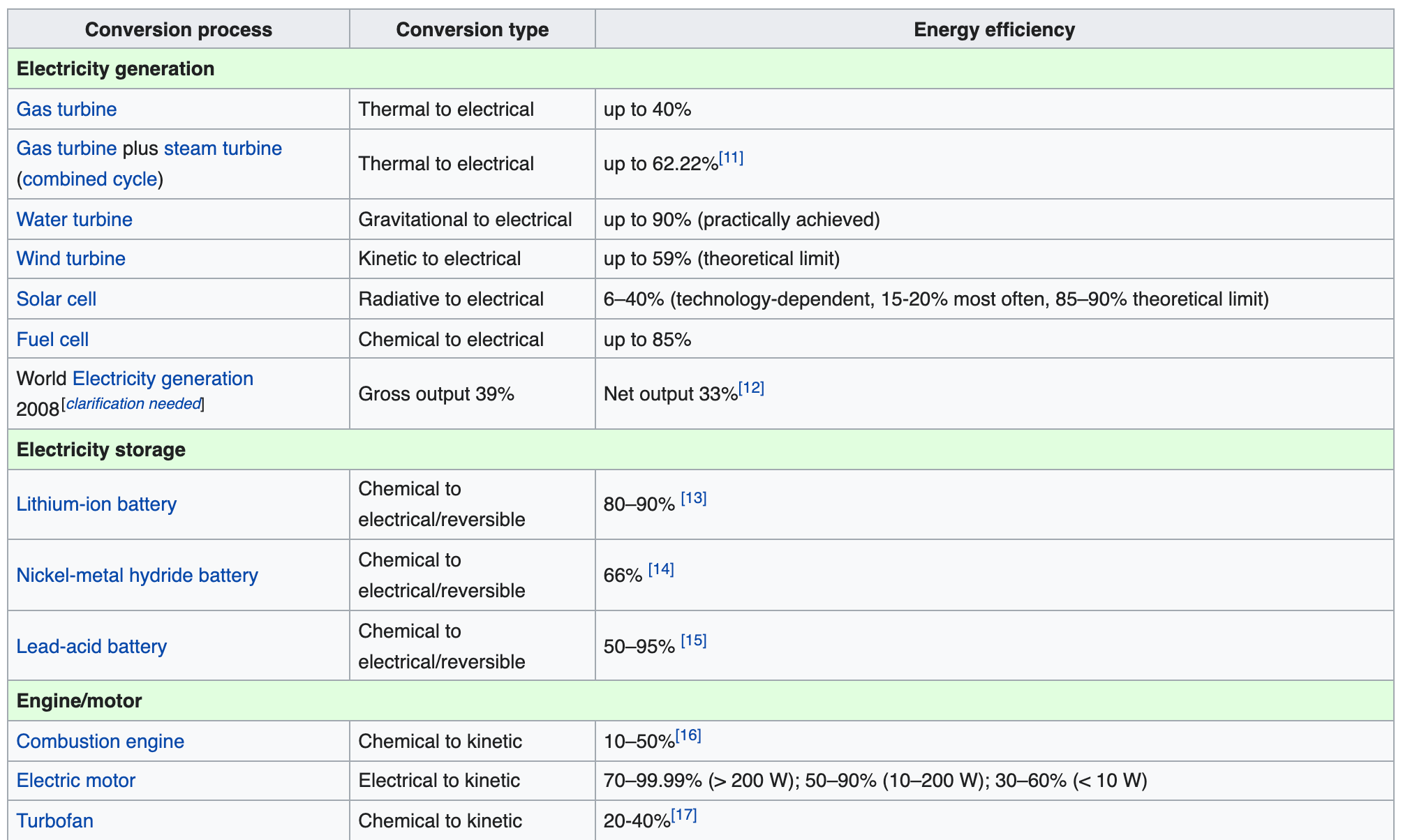
- Isothermal heat transfer: hot reservoir to working fluid at \(T_h\).
- Adiabatic expansion of working fluid to \(T_c\).
- Isothermal heat transfer: working fluid to cold reservoir at \(T_c\).
- Adiabatic compression of working fluid to \(T_h\).
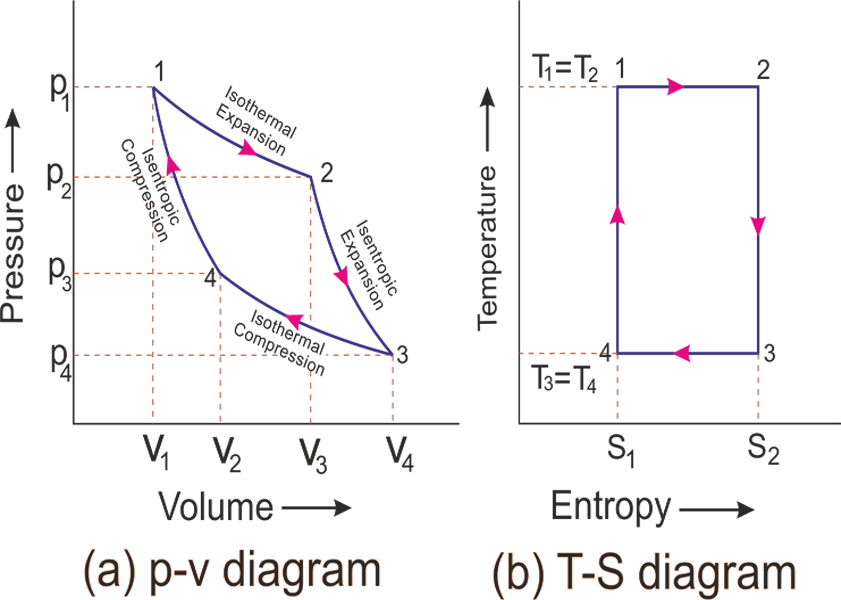
Carnot efficiency \(\eta_c = 1-\frac{T_c}{T_h}\)
Otto Cycle
- Gas power cycle (uses a gas as the working fluid)
- Spark ignition engines (gasoline)
- The working fluid is not fixed (intake, exhaust)
- Four strokes:
- Intake
- Compression
- Expansion
- Exhaust

Otto Cycle: steps
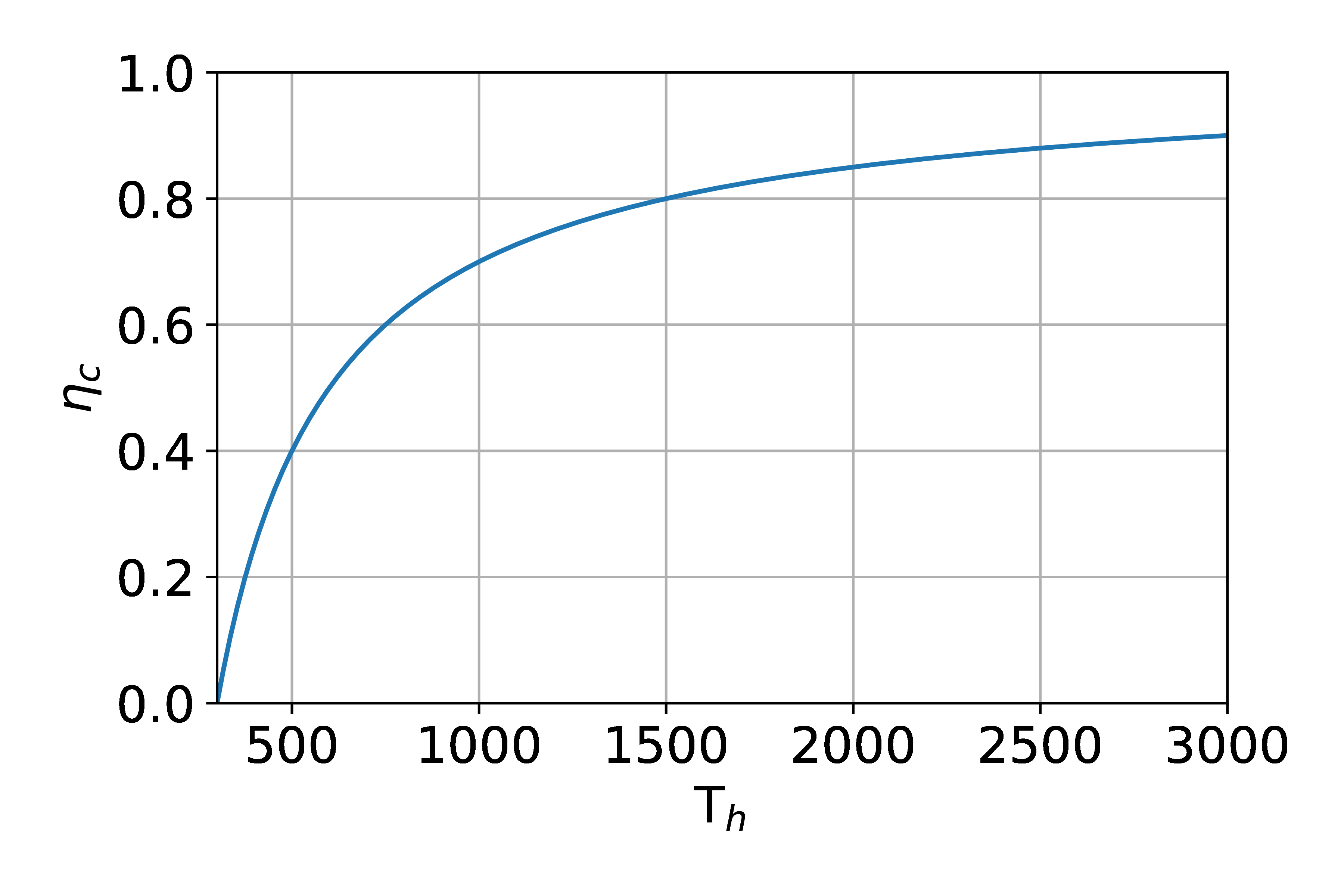
- Steps
- 0-1: Intake, const P
- 1-2: \(W_{in}\): Adiabatic compression
- 2-3: \(Q_{in}\):
Heat addition, combustion
- fast: constant V
- 3-4: \(W_{out}\): Adiabatic expansion
- 4-1: \(Q_{out}\):
Heat rejection, valve open
- fast: constant V
- 1-0: Exhaust
- How do these correspond to the 4 strokes?
- Note, the area under the PV diagram is the work; the area under the TS diagram is the heat.
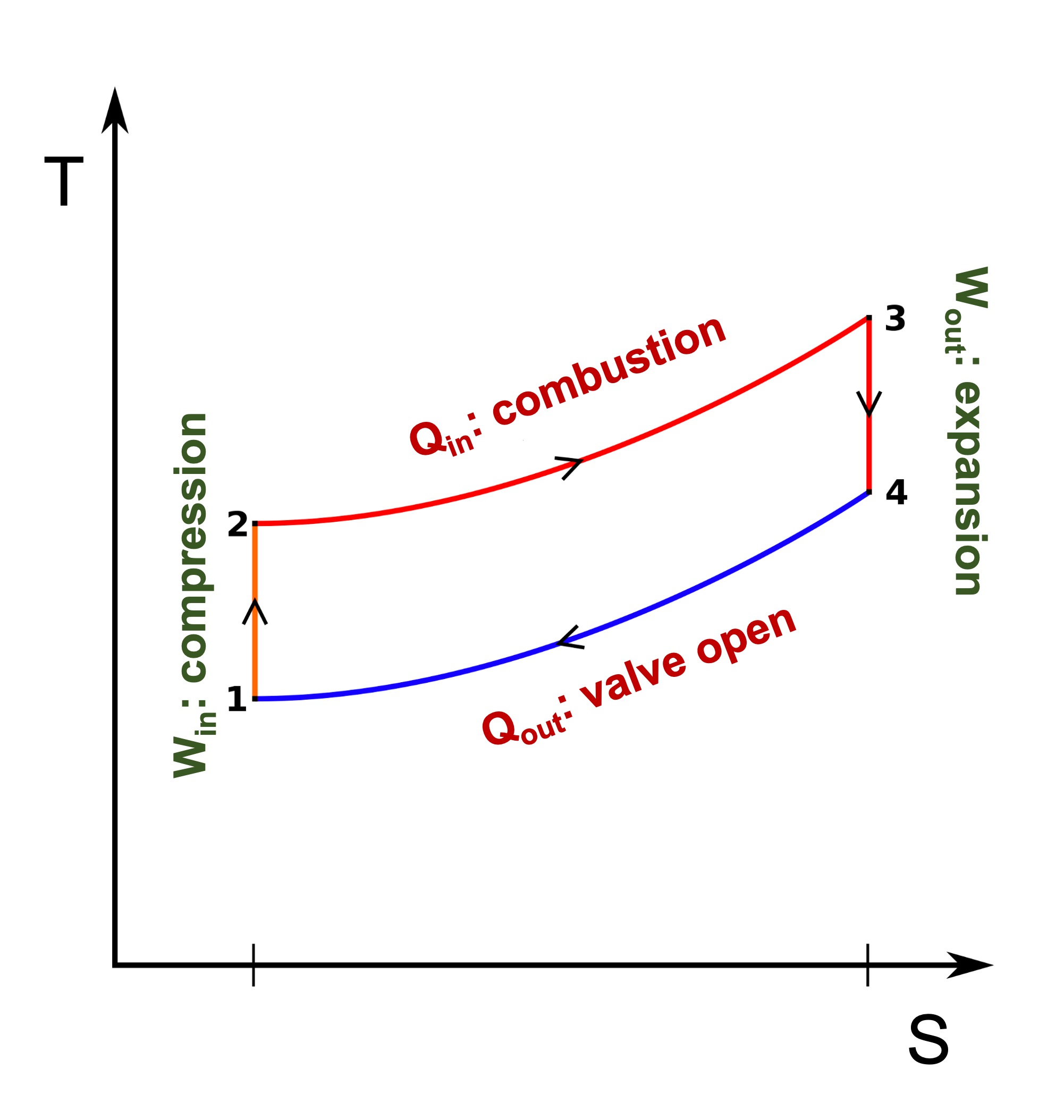
Otto cycle: net work
Net work is area under curve 3-4 minus area under curve 1-2
Question: how to best compute the net work?
Write the net work in terms of the internal energies at points 1, 2, 3, 4.
- The compression and expansion steps are adiabatic.
- \(W_{in} = u_2-u_1\)
- \(W_{out} = u_3-u_4\)
- \(W_{net} = W_{out}-W_{in} = (u_3-u_4) - (u_2-u_1)\)
- there is a simplification here for combustion…

Adiabatic compression/expansion
Otto cycle: efficiency
- Assume air as a working fluid
- Assume constant heat capacity
\[η = \frac{W_{net}}{Q_{in}}\] \[W_{net} = (u_3-u_4) - (u_2-u_1)\] \[W_{net} = c_v(T_3-T_4) - c_v(T_2-T_1)\] \[Q_{in} = u_3 - u_2 = c_v(T_3-T_2)\] \[η = \frac{T_3 - T_4 - T_2 + T_1}{T_3-T_2} = 1 - \frac{T_4 - T_1}{T_3 - T_2}\] Factor out \(T_1\) from the numerator and \(T_2\) from the denominator: \[η = 1 - \left(\frac{T_1}{T_2}\right)\left(\frac{T_4/T_1 - 1}{T_3/T_2 - 1}\right)\]
Otto cycle: efficiency
\[η = 1 - \left(\frac{T_1}{T_2}\right)\left(\frac{T_4/T_1 - 1}{T_3/T_2 - 1}\right)\] Now, the compression and expansion steps are adiabatic, so \(TV^{γ-1}=\mbox{const}\). Hence,
\[η = 1 - \frac{1}{r^{γ-1}}\]

How does this efficiency compare to Carnot?
Diesel cycle

Diesel cycle: thermodynamics
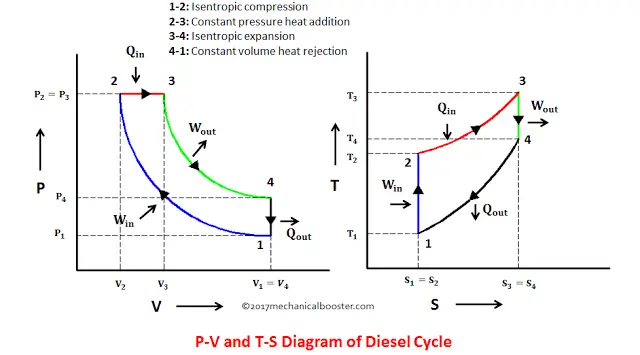
Key difference with the Otto cycle is the constant pressure heat addition rather than constant volume.
Diesel cycle: efficiency

Question: do you expect the efficiency of the Diesel cycle to be higher or lower than for the Otto cycle?
\[η = 1 - \frac{1}{r^{γ-1}}\left(\frac{α^γ-1}{γ(α-1)}\right),\] where \(r=V_1/V_2\) is the compression ratio and \(α=V_3/V_2=T_3/T_2\) is the cut-off ratio. \(\alpha\) can be written in terms of the high and low temperatures and the compression ratio: \(\alpha=T_3/(T_1r^{\gamma-1})\).
Brayton cycle: gas turbines
Brayton cycle: thermodynamics

- Gas turbine combustors
- Like the Otto cycle, but heat transfer steps are constant \(P\) instead of constant \(V.\)
- Same efficiency as the Otto cycle, but often written in terms of the pressure ratio.